
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the isomeric relationship ?
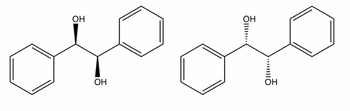
Transcribed Image Text:Ž......
НО
.......
OH
Expert Solution
arrow_forward
Step 1
We have given two compounds and we have to identify isomeric relationship between them.Chiral centre can be defined as the carbon atom with which four different groups are attached.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- what is the oxidizing agent in IO3 + Re --> ReO4 + IO-arrow_forward4. Consider [Fe(H2O)]Clz. The bonding between hexaaquairon(III) and chloride is best described as: (a) metallic (b) ionic (c) covalent (d) coordinate covalentarrow_forward4. This question concerns the d-block elements, Sulphur, Nitrogen and Group IV elements,. (a) (i) Distinguish between a d-block element and a transition metal. (ii) Write down the symbols for the elements of the first transition series in increasing order of atomic number. (iii) State the property of the transition element which enables them to act as catalyst in many industrial processes.arrow_forward
- The Iron Triad is a historical name for the group of elements Fe, Co, and Ni. It was used to refer to these elements in the same period that share similar chemical and physical characteristics. All three elements in the Iron Triad can form +2 and +3 ions. For each element: a) Write the neutral, +2 oxidation state, and +3 oxidation state electron configuration using the condensed noble gas notation. Indicate which ions in the group are isoelectronic. b) Indicate the number of unpaired electrons in the +2 and +3 oxidation state and whether each ion is diamagnetic or paramagnetic.arrow_forward2. Water has a dielectric constant of 80. How does this explain why most salts dissolve in water?arrow_forwardBalance the following chemical equation (if necessary): Ca(C2H3O2)2(aq) + Na:CO:(aq) – CaCOs(s)'+ NaC:H;Oz(aq) 2-3-O4+arrow_forward
- What is the name of the compound that forms when Cu+ combines with SO32- ? You can use either the accepted IUPAC Roman numeral names or the old Latin names for the metal.arrow_forwardFictional, thus not on the periodic table. Lz, a transition metal, forms the compound LzS₂ when bonded to sulfur. What would be the formula of Lz in that same oxidation state bonded to nitrogen? Period 4 11 H Li Na 19 2641 LZ4N Group S 5 Rb Sr Y 38 2016-1 151500 TOWN Lz₂N 3 Be LZN ₂ LzN Mg **Denotes the presence of (2-8-) for elements 72 and above Lz3 N4 20 2442 Cs Ba La Hf 55 20wwe 2w1982 Lz3N2 KEY 12 4 WWWO 140 M K Ca Sc Ti V 21 23 Nb Mo Tc Ru Rh Pd Ag Cd In Ru Rh 48 49 DISIN +00.22 3. Ta W Re Os Ir Pt Hove 15-11-2 18-32-18-2 22 25192 Zr Atomic Mess12.011 Electron Configuration Acomic Number 6 2-4 With Oxidation Numbers 1407 Symbol 58 5 90 7 Fr Ra Ac Rf Db Sg Bh Hs Mt Uun Uuu Uub 111 112 21531592 Ce 6 C Group 7 8 38M 200 Cr Mn Fe Co 24 16132 56142 Th Pa 91 Selected Oxidation State Relative atomic masses are based on ¹40-12.000 Note: Mass numbers in parte are mass numbers of the most stable or common isotope. MOH 52 U 9 10 11 12 Ni Cu Zn Ni Cu Zn 16162 KE 14 BCN Al Si P BUT 13 Ga Ge…arrow_forwardGive 5 behavior and properties of metallic bondingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY