
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
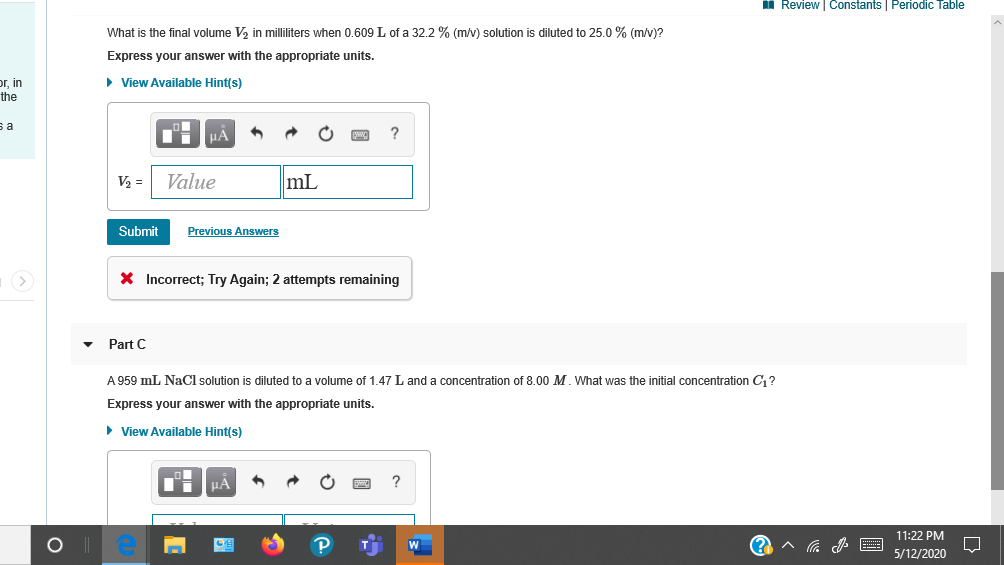
Transcribed Image Text:What is the inal volume Va in milhiters when 0.s09 L of a 322 % Imv) solution is diluted to 25.0% imv?
Express your answr with the appropriate units.
• View Availsble Hintjs)
V = Value
mL
Submil
Previous Answa
X Incorrect; Try Again; 2 attempts remaining
Part C
A 959 mL. NaCl solufon is diuled to a volume of 1.47 L and a concentrsion of 0.00 M. What was the intial concentrstion C?
Express your answer with the appropriate units.
• View Available Hints)
Expert Solution
arrow_forward
Step 1
The dilution equation is used to find the final volume, V2 as follows,
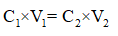
arrow_forward
Step 2
Convert 0.609 L into mL
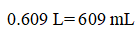
The unknown volume is found as follows,
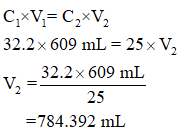
Therefore, the final volume, V2 = 784.392 mL
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- E D Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution contains 573 g NaOH(s) per liter of solution. Calculate the molarity of this saturated NaOH(aq) solution. concentration: 80 $ 4 R gog F4 F V % LO 5 FS T G A 6 B MacBook Air F6 Y H & 7 N 44 F7 U * 00 8 M Question Source: McQuarrie, Rock, And Gallogly 4e-General Chemistry | Publisher: University Science Books Pi Pll FB T MOSISO K ( 9 DD FO H O presented by Macmillan Learning ) 0 command F10 P || B 180 F11 option + 11 4) F12 M } delete ret 8arrow_forwardA 35.0 mL volume of ammonia (NH3) household cleaner needs 53.75 mL of 0.300 M HNO3 to completely neutralize the base. The balanced equation is: HNO3 + NH3 --> NH4NO3 A) Find the number of moles of HNO3 involved in this reaction. (2 hints: start dimensional analysis with the volume of HNO3, & convert mL to L.) B) Find the molarity (M) of the NH3 (This is different than the number of moles. Your calculation will be different from part A.) C) Find the mass/mass % of the NH3. You will need the molar mass of NH3 (17.04 g/mol) & the density of NH3 (0.985 g/mL).arrow_forwardezto.mheducation. Mc McGraw-Hill Education Campus ductory Chemistry I (lec) (CHEM-1305-7Z3), Topic: Unit 6:.. Saved er 6 Homework i Example 9 - Dilution - use molarity and volume (mL) values to calculate vo Calculate the volume, in L, of water that must be added to dilute 24.6 mL of 10.6 M HCl to 0.105 M HCI. Enter your answer in the provided box. L water ces MAR 1 21 étv MacBook Airarrow_forward
- nok Experiment 1: Laboratory Report Tixed Salt Solutions 4. For ench renction! A> urite a word equntion b) vrite the balanced moleculas eauntion c) write the total ced J) vritc the balmced net ionic enuntron ionsc enuntron 1, Renctants.' Ag Noz caz) NazCOz cra) 2. Renctmts i Tese co CNOZ)2 Na z S cn> 3. Renctats ; cuCNOg)2arrow_forwardShow ghe complete detail of solutionarrow_forwardto click suf our answers before submitting. DQuestion 27 Determine the molarity (M) of a solution formed by dissolving 682.3 g of Mgl2 in enough water to yield 588.5 mL of solution. Next e Drive * Previous Submi Not saved MacBook Air 80 F8 esc F5 F6 F7 F3 F4 F1 F2 #3 & 2$ 4 @ 7 9. W E R Y tab S F caps lock Z shift C V * 00arrow_forward
- Chapler 6 Tost Redo lI) 6.531 9 sodium hydrox.de soluton are added. to 8.426 g of Tron () chloride solutim. How much iron() hydronde precipitale should be produced? If 4.7329 uas achually produced, what was the % yield ? 2. In the same reachion, it 250 ml o a 03 Somol/L solutin Fe Cls la) was combined with" 5.9639 of sodium hydroride id'a soluhim, ihow much precipitate is formed?arrow_forwardplease show all work . thank youarrow_forwardI need both 2a and 2 barrow_forward
- uton aDunat is tho molarHy of agurose solittan that cantainS 100gof (190.19 a mol) dissonod in Tooi O ml ८ ेण 2' molarty of adlu cose Cipttig Ooarrow_forward5) If all the NaHCO3 in bag 2 reacted, calculate the number of moles of gas produced? tates) E Focusarrow_forwardActivity 2. Complete the table to prepare the given solutions with different concentrations: Mass Solute Volume of Water to be added Solutions Volume Solute (if liquid A. 5 % by volume, 25 mL HNO, solution NG PA PASIG OF 1S73 B. 1.5 molar, 50 ml nitric acid solution C. 20%, 50 ml Naci solution NOISIA Iarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY