
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What is the hybridization of each N atom and does their lone pairs participate in resonance?
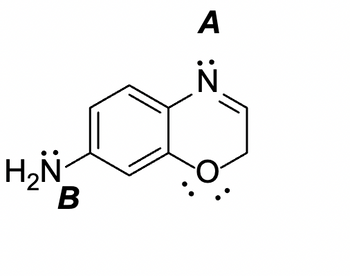
Transcribed Image Text:H₂N
B
A
Z: D
Expert Solution
arrow_forward
Step 1
for the given structure, we have to determine if the lone pair of N atom A and N atom B participate in resonance and the hybridization.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- + H₂N. ²HN.arrow_forwardC₁H₁₂O₂ The molecular formula of an unknown compound is C,;H,,0,. (SH,m), 1.4 8 (6H, d), 5.2 3 (1H, septet). Identify the unknow organic compound? 2.The compound gives strong peak at1745 cm 1 in IR spectrum. The NMR details are 7.55 8arrow_forwardHCl (excess) H₂O₂arrow_forward
- Whay are the products. Explainarrow_forwardFor each of the following, identify the product (represented by A and B) that would be formed through the indicated sequence of steps from the given starting material. Show ALL the products' structures along the way for each step.arrow_forwardOH conc. H₂SO + + +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning