
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
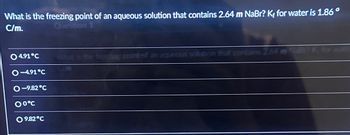
Transcribed Image Text:What is the freezing point of an aqueous solution that contains 2.64 m NaBr? Kf for water is 1.86°
C/m.
Question
4.91°C
-4.91°C
O-9.82°C
00°℃
9.82°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the freezing point of 0.920 M aqueous acetic acid? The density of this solution is 1.008 g/cm3. Ka for acetic acid is 1.7x10^-5.Kf for water is 1.858\deg C/m. Tf=arrow_forwardTrue or false: If a sodium hydroxide solution is stored in a glass bottle the concentration of hydroxide will increase over time.arrow_forward26. The osmotic pressure of a MgSO4 (120.4 g/mol) solution at 25°C is 0.489 atm. Calculate the molarity of the MgSO4 solution. A) 0.01 MB) 0.02 M C) 0.03 M D) 0.04 Marrow_forward
- What is the freezing point of a solution of 0.208 moles of the nonelectrolyte urea (60.06g/mol) in 600.0g of benzene.(The normal freezing point of benzene is 5.50 oC and Kf = 5.12 oC/m) Select one: a. 1.77 oC b. 3.73 oC c. 7.27 oC d. 1.06 oC e. 4.44 oCarrow_forwardI Review | Constants | Periodic Table Determine the volume, in milliliters, required to prepare each of the following diluted solutions: Part A 259 mL of a 0.200 M HNO3 solution from a 4.00 M HNO3 solution Express your answer with the appropriate units. HA ? Value Units Submit Request Answer Part B 713 mL of a 0.100 M MgCl, solution using a 6.50 M MgCl, solution Express your answer with the appropriate units. µA ? Value Unitsarrow_forwardA high school chemistry teacher is preparing a solution by dissolving, 17.5mL of ethanol (ETOH) in H20 to make 145mL of total solution. The molar mass of Ethanol is 46.07 g/mole, and the density of EtOH is 0.789 g/mL. Determine the concentration (in M) of the ethanol in this solution?arrow_forward
- The freezing point of an aqueous solution containing 35 g of an nonelectrolyte in 150 mL water is -10.4ï°C. What is the molecular weight of the compound? Kf = 1.86°C/m for water. 121 g/mol 2.78 g/mol 34.4 g/mol 41.73 g/mol 53.8 g/molarrow_forwardPlease don't provide handwritten solution.....arrow_forwardWhat is the freezing point of an aqueous solution that boils at 105.0°C?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY