
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
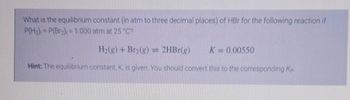
Transcribed Image Text:What is the equilibrium constant (in atm to three decimal places) of HBr for the following reaction if
P(H₂) = P(Br₂), = 1.000 atm at 25 °C?
H₂(g) + Br₂(g) = 2HBr(g) K = 0.00550
Hint: The equilibrium constant, K, is given. You should convert this to the corresponding Kp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At 1359 °C the equilibrium constant for the reaction: 2 BrCl(g) = Br₂(g) + Cl₂(g) is Kp = 2.11. If the initial pressure of BrCl is 0.00467 atm, what are the equilibrium partial pressures of BrCl, Br₂, and Cl₂? p(BrCl): = p(Br₂) = p(Cl₂): =arrow_forwardWhen heated, ammonium Carbonate decomposes according to the reaction: NH4CO2NH3(s) ⇌ 2NH3(g)+CO2(g). At 450C, the total pressure of the system at equilibrium was 0.318 atm. What is Kp for the reaction at this temperature?arrow_forwardPlease don't provide handwritten solution .....arrow_forward
- Consider the following equilibrium process at 686. °℃: CO₂(g) + H₂(g) CO(g) + H₂O(g) The equilibrium concentrations of the reacting species are [CO]=0.0560 M, [H₂] = 0.0400 M, [CO₂]=0.0830 M, and [H₂O]=0.0430 M. Part 1 of 5 Calculate K for the reaction at 686. °C. Round your answer to 3 significant digits. с Kc- Part 2 of 5 x10 If we add CO₂ to increase its concentration to 0.510 M, what will the concentrations of all the gases be when equilibrium is reestablished? Round your answer to 3 significant digits. [CO₂] = M x10 X Śarrow_forwardAt 1000 K, a sample of pure NO₂ gas decomposes: 2 2NO₂(g) = 2NO(g) + O₂(g) The equilibrium constant Kp, is 158. Analysis shows that the partial pressure of O₂ is 0.41 atm at equilibrium. Part 1 of 2 What is the pressure of NO? Be sure your answer has the correct number of significant digits. Part 2 of 2 atm x10 atm X What is the pressure of NO₂? Be sure your answer has the correct number of significant digits. Ś x10arrow_forwardConsider the equilibrium system described by the chemical reaction below. At equilibrium, a sample of gas from the system is collected into 5.00 L flask. The flask is found to contain 8.62 g of CO, 2.60 g of H2, 43.0 g of CH4, and 48.4 g of H:O at 320.0 °C. What are the values of Kc and Kp for this reaction? CH:(g) + H2O(g) = CO(g) + 3 H2(g) 1 2 NEXT > Based on the given data, set up the expression for Kc and then evaluate it. Do not combine or simplify terms.arrow_forward
- Consider the following equilibrium: N2O4(g) 2NO2 (g) AG = 5.4 kJ Now suppose a reaction vessel is filled with 2.78 atm of dinitrogen tetroxide (N204) at 710. °C. Answer the following questions about this system: rise x10 fall Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO2 needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm X Sarrow_forwardAt -5.21 °C the concentration equilibrium constant K = 2.9 × 10³ for a certain reaction. Here are some facts about the reaction: • The net change in moles of gases is -1. Some of the reactants are liquids and solids. • If the reaction is run at constant pressure, 58.0 kJ/mol of heat are released. ● Using these facts, can you calculate Kat 16. °C? If you said yes, then enter your answer at right. Round it to 2 significant digits. If you said no, can you at least decide whether Kat 16. °℃ will be bigger or smaller than Kat -5.21 °C? Yes. No. 0 Yes, and K will be bigger. Yes, and K will be smaller. No.arrow_forwardHeating HI(g) at 425 °C causes some of this compound to decompose, forming H2 (g) and I2 (g). Eventually, the amounts of the three species do not change further, the system has reached equilibrium. (At this point, approximately 22% of the HI has decomposed.) What is happening in this system at the molecular level? O H2(g) reacts with I2 (g) to produce HI(g) at the same rate as it decomposes. H2 (g) and I2 (g) molecules collide with HI(g) making the reaction stop. Only part of the HI(g) molecules has enough energy to decompose at this temperature. 5:55 PM FLV 2/22/2020 Type here to search hp ins prt sc delete home f12 f8 f9 f10 f5 f6 f7 14 10 num & backspace lock 23 8. 3 %24arrow_forward
- 4. Toluene, C;H8(1), is an important organic solvent. It is made industrially from methylcyclohexane, C,H14(g): C,H14(g) + heat ? 2C;H8(1) + 3H2(g) State three different changes to an equilibrium mixture of these reacting gases that would shift the reaction toward greater production of toluene. State and explain each change.arrow_forwardConsider the following equilibrium: N,0, (g) = 2NO, (3) AG' = 5.4 kJ Now suppose a reaction vessel is filled with 0.496 atm of nitrogen dioxide (NO,) at 137. °C. Answer the following questions about this system: O rise Under these conditions, will the pressure of NO, tend to rise or fall? O fall Is it possible to reverse this tendency by adding N,O,? In other words, if you said the pressure of NO, will tend to rise, can that O yes be changed to a tendency to fall by adding N,04? Similarly, if you said O no the pressure of NO, will tend to fall, can that be changed to a tendency to rise by adding N,0,? If you said the tendency can be reversed in the second question, calculate the minimum pressure of N,0, needed to reverse it. O atm Round your answer to 2 significant digits.arrow_forwardConsider the following equilibrium: N₂ (g) + 3H₂(g) → 2NH3(g) AG = -34. KJ Now suppose a reaction vessel is filled with 3.40 atm of nitrogen (N₂) and 4.50 atm of ammonia (NH3) at 250. °C. Answer the following questions about this system: Under these conditions, will the pressure of NH3 tend to rise or fall? Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH3 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. OO rise fall yes no atm ☐x10 X Śarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY