
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
What is the energy of a photon with a frequency of 7.47 × 10¹⁴ s⁻¹? (h = 6.626 × 10⁻³⁴ J • s)
Expert Solution
arrow_forward
Step 1
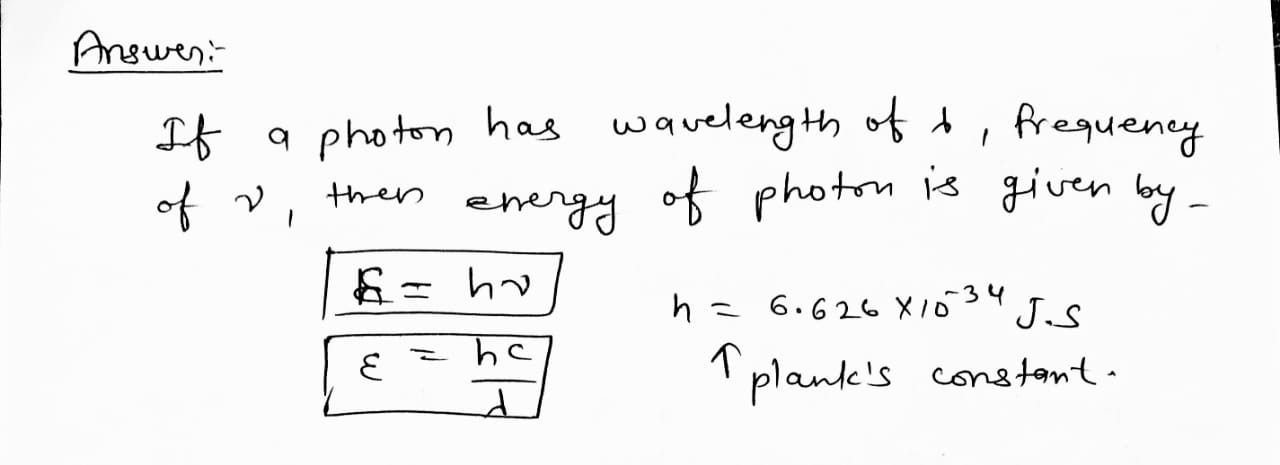
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the energy (in J) of a photon with a frequency of 7.23 × 10¹⁴ s⁻¹? (h = 6.626 × 10⁻³⁴ J • s)arrow_forwardIn Einstein's photoelectric experiment, the kinetic energy of an electron displaced from a metal by a photon was the difference between the energy of a photon and a threshold energy value for the metal. If you were to perform a similar experiment with the metal magnesium, which has a threshold energy of 5.86 × 10⁻¹⁹ J, and ultraviolet light of wavelength 55.5 nm, what would be the kinetic energy (in J) of the electron displaced?arrow_forwardThe scattering of sunlight by the mixture of gas molecules in air results in the blue color of the sky. Calculate the energy (in Joules) of a single photon of blue light with a frequency of 7.50 × 1014 Hz. (h = 6.626 x 10-34 J•s) |x 10 49 J 2 3 4 5 C 7 8. +/- х 100arrow_forward
- What is the wavelength (in nm) of a photon if the energy is 5.51 × 10⁻¹⁹ J? (h = 6.626 × 10⁻³⁴ J • sarrow_forwardWhat is the wavelength (in nm) of a photon if the energy is 6.21 × 10⁻¹⁹ J? (h = 6.626 × 10⁻³⁴ J • s)arrow_forwardDetermine the energy, in J, of a photon with a wavelength of 351 nm. (h = 6.626 × 10⁻³⁴ J • s and c = 3.00 × 10⁸ m/s)arrow_forward
- A photon has a frequency of 8.37 × 10⁸ Hz. What is the energy of this photon in Joules?arrow_forwardA hydrogen atom absorbs a photon with a wavelength of 1094 nm and causes an electron to make a transition from an unknown n1 to a final n2 = 6. Part A Calculate frequency of the absorbed photon in units of s-1. Part B Calculate the energy change in the atom (ΔEatom in J) upon absorption of the photon. Part C Calculate the value of n1arrow_forwardDetermine the energy of a photon with a wavelength of 317 nm. (h = 6.626 × 10⁻³⁴ J • s and c = 3.00 × 10⁸ m/s)arrow_forward
- A certain shade of blue has a frequency of 7.13×1014 Hz. What is the energy of exactly one photon of this light? Planck's constant is ℎ=6.626×10−34 J⋅s.arrow_forwardWhat is the energy (in J) of a mole of photons that have a wavelength of 503 nm? (h = 6.626 × 10⁻³⁴ J • s and c = 3.00 × 10⁸ m/s)arrow_forwardCalculate the energy (in J) of photons with frequency of 27.3 MHz 2.21 x 1014 1.81 x 10-19 O 1.81 × 1026 1.12 x 107 1.12 x 1014arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY