Question
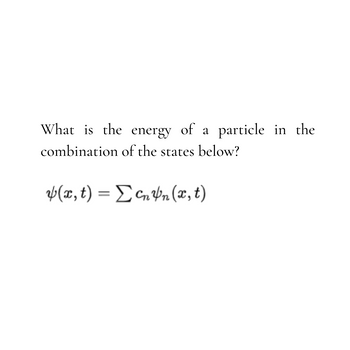
Transcribed Image Text:What is the energy of a
combination of the states below?
(x, t) = ΣcnYn (x, t)
particle in the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- The difference in energy between allowed oscillator states in H2O molecules is 0.454 eV. What is the oscillation frequency (in Hz) of this molecule? Hzarrow_forwardwave function Find the anplitude A of the above =xp(品). %31 7. 77 UIS יר 11 Sin 7. 7. UIS o(x)=A sin (ax) free particle - having particle Electron can be considered a s and Wave function int, Well Hn electron traparrow_forwardThe energy spectrum of a bound state is None of the above Discrete O Continuous but with some missing lines Continuousarrow_forward
- An electron in its ground state is trapped in the one-dimensional Coulomb potential energy. What is the probability to find it in the region between x = 0.92a and x = 1.08ao? Additional Materials eBookarrow_forwardelectron with mass m moring inside box 0LXLa, if function is given by: you. know tkut te Yos=2 sin what is tu state of tu electron and what is tu energy of electrenarrow_forwardA Proton is confined to move in a one- dimensional bux of length 0.410 m a) Find the lowest possible energy of the proton. Note: Answer must be in evarrow_forward
arrow_back_ios
arrow_forward_ios