Question

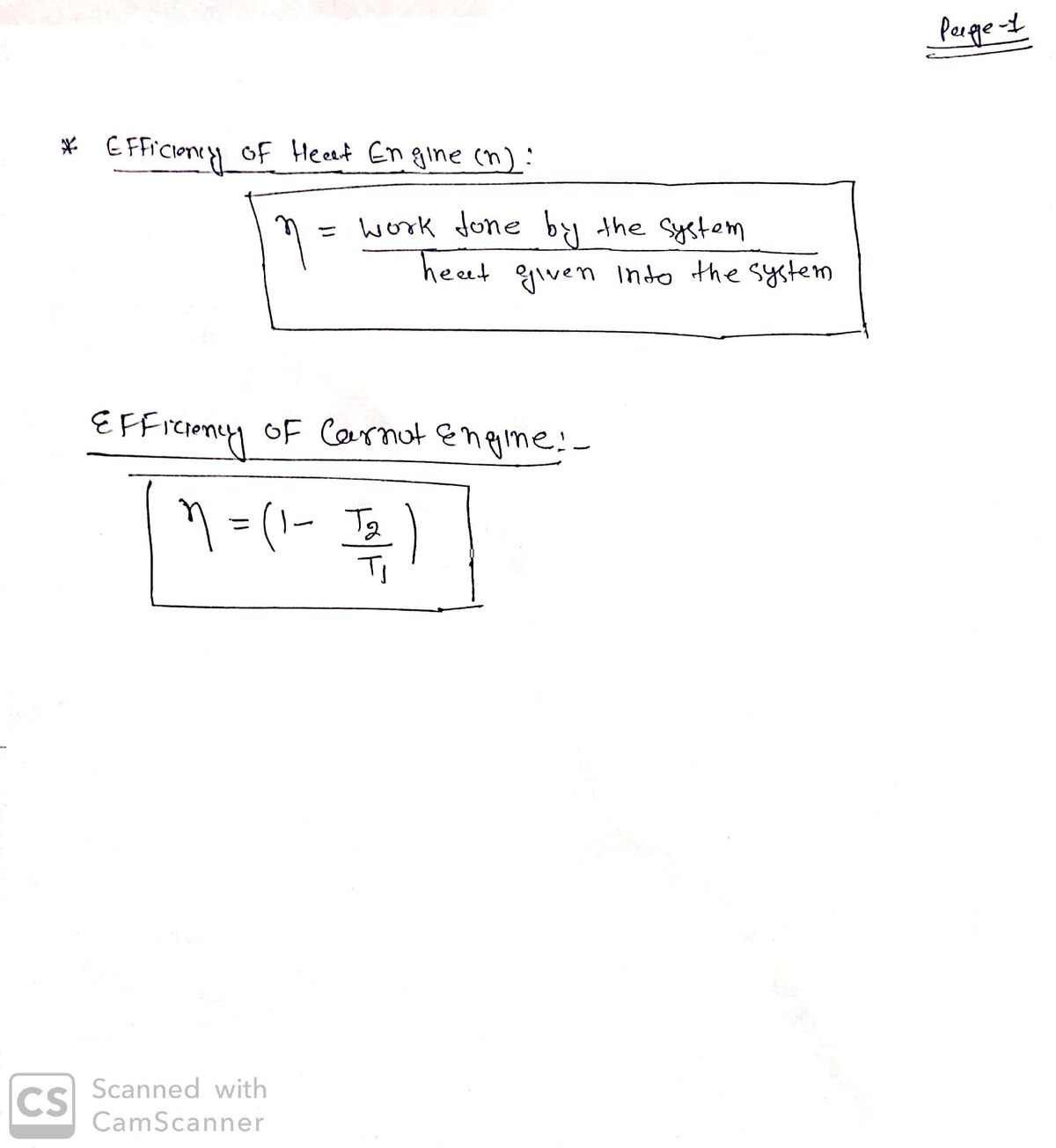
Transcribed Image Text:What is the efficiency of a Carnot engine whose isothermal process happen at 30.0 degC and 218 degC?
Number
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- A particular heat engine has a mechanical power output of 4.20 kW and an efficiency of 26.0%. The engine expels 8.15 103 J of exhaust energy in each cycle. (a) Find the energy taken in during each cycle. KJ (b) Find the time interval for each cycle. sarrow_forwardCalculate the net work output of a heat engine following path ABCDA in the figure below, where V1 = 2.6 ✕ 10−3 m3 and V2 = 10.4 ✕ 10−3 m3.arrow_forwardA particular heat engine has a mechanical power output of 5.20 kW and an efficiency of 26.0%. The engine expels 7.40 x 103 J of exhaust energy in each cycle. (a) Find the energy taken in during each cycle. kJ (b) Find the time interval for each cycle. Sarrow_forward
- The efficiency of all real engines are less than that of the carnot engine. Why?arrow_forwardQUESTION 4 In a single cycle, heat engine extracts 8.00 kcal of heat from a hot reservoir and exhausts 5.00 kcal of heat int a cold reservoir. What is the efficiency of this engine? а. 67.5% b. 77.5% C. 57.5% d. 47.5% е. 37.5%arrow_forward
arrow_back_ios
arrow_forward_ios