Question
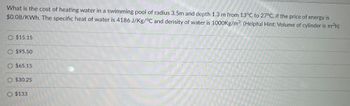
Transcribed Image Text:What is the cost of heating water in a swimming pool of radius 3.5m and depth 1.3 m from 13°C to 27°C, if the price of energy is
$0.08/KWh. The specific heat of water is 4186 J/Kg/°C and density of water is 1000Kg/m³. (Helpful Hint: Volume of cylinder is 7²h)
O $15.15
O $95.50
O $65.15
O $30.25
O $133
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images
