
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
kindly help me with this problem thank you :)
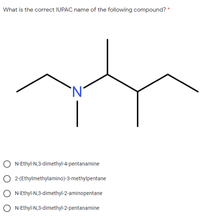
Transcribed Image Text:What is the correct IUPAC name
of the following compound? *
`N'
N-Ethyl-N,3-dimethyl-4-pentanamine
2-(Ethylmethylamino)-3-methylpentane
N-Ethyl-N,3-dimethyl-2-aminopentane
N-Ethyl-N,3-dimethyl-2-pentanamine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Make a graph of the following data points. Is the relationship linear? If so, find the slope and y-intercept. Are the variables directly proportional?voltage (V) 1.01.52.02.53.03.54.0 current (A)3.45 x 10-35.02 x 10-36.88 x 10-38.60 x 10-31.01 x 10-21.22 x 10-21.36 x 10-2 Linear?_______________Slope_________________Y-Intercept____________Directly proportional?____arrow_forward12:50 ← d2fcc1b0-17ba-42ff-a... 15/24 SCIENCE (086) 9 જે SCIENCE 224 Science Project Create a comprehensive and informative project on ' Natural resources' in the form of an artistically crafted, decorated project file Include pictures, illustrations,examples, surveys, advertisements, newspaper cuttings and headings etc in your project to make your project appealing, relevant, easy to understand and memorable Keypoints . Introduction to Natural Resources • Air pollution and its causes Water pollution and its causes Soil pollution and its causes . Biogeochemical Cycles 1. Oxygen Cycle 2. Carbon Cycle 3. Nitrogen Cycle 4. Water Cycle Rain and effect of acid rain . Green-house Effect Ozone Layer and reason for ozone depletion Assignment Sheet 1.Look at Fig. 1.1 and suggest in which of the vessels A,B, C or D the rate of evaporation will be the highest? Explain. PDF to Long Images PDF to Images =arrow_forwardYou have just started working in an Urgent Care Clinic as a nurse. Mom brings in her 3 year old little boy, Brandon. Mom states that Brandon has been having fever up to 104F and he isn't eating or drinking anything because he feels very sick to his stomach, not to mention crabby! He's also been pulling a lot at his right ear and sounds congested. At the last well visit with the Pediatrician (about 2 weeks ago), Mom states that Brandon weighed 32 pounds. On exam today, his weight is 12.5 kg. Additionally his eyes are darkened and sunken, his mouth is dry with some white crusting around the lips. His temperature is 39C, other vitals signs are normal. Though he looks ill he appears to be medically stable. The doctor rushes in, quickly exams Brandon, diagnoses him with otitis media and then barks off orders to you that you then need to convey accurately to Mom. The orders include: push fluids, ibuprofen 10mg/kg every 6 hours if temp > 38C, and begin taking a 10-day course of…arrow_forward
- 1 4 6 7. 8 9. 10 -10 An aqueous solution at 25 °C has a H,O' concentration of 6. x 10 "M. Calculate the OH concentration. Be sure your answer has 1 significant digits. | M Subm Continue © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centarrow_forwardSelect one for each boxarrow_forwardLearning AA prod03-cnow-owl.cengagenow.com Login Learning Learning × Online tea... y dr. marlow... ember L... with We 3. AS surroundi... M 2BrF3 (9) Br2 (g) + 3F2 (9) 4. AG° = AH°... M 5. AG: Pre... 1req 6. AG: Enthal... Using standard thermodynamic data at 298 K, calculate the free energy change when 2.14 moles of BrF3 (9) react at standard conditions. Substance AG (kJ/mol) 7. AG fro... 1req Question Question Question 8. Calculat... 1req AG° = 9. Calculat... 1req BrF3 (9) -229.4 Br2(g) 3.1 F2(9) 0.0 kjarrow_forward
- 1-Pentanol to 1-bromopentane Chemicals: - 60ml Conc. Sulfuric Acid - 100ml Saturated Sodium bicarbonate - 65ml 1-Pentanol - 78g sodium bromide - Distilled water - 58.42g 1-Bromopentane 1-Pentanol Sodium Bromide Sulfuric Acid 1-Bromopentane Formula C5H12O NaBr H2SO4 C5H11Br MW (g/mol) 88.15 102.894 98.078 151.04 Density (g/mL) 0.811 3.21 1.84 1.218 Boiling point (*C) 138 1,396 337 130 NaBr(aq) + H2SO4(aq) -> NaHSO4(aq) + HBr(aq) CH3(CH2)4OH(aq) + H+ Br- (aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4Br(aq) + H2O(aq) How do I calculate the percent yield and identify the limiting reagent?arrow_forwardVolume of acetic anhydride: 2.98 mL Mass of salicylic acid: 1.00 g Mass of aspirin recovered: 0.67 g Show that mmol of aspirin recovered divided by mmol of limiting reagent x 100% gives the same % yield (please explain) Thank you.arrow_forward6. While cleaning you find out that you unfortunately only have run out of your usual cleaning solutions. Your friend thankfully has the cleaner you want, but not at the dilution you want. If their solution is at a 1/15 dilution, devise a serial dilution scheme to get a 1/30, 1/60, and 1/180 dilution as a result.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY