
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give correct detailed Solution with explanation needed..please avoid handwritten Solution
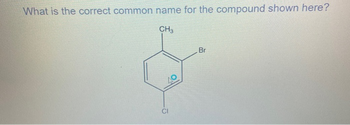
Transcribed Image Text:What is the correct common name for the compound shown here?
CH3
CI
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please don't provide handwritten solution..arrow_forwardd Tap - Cengage Learning vo/index.html?deploymentld%3D55750828934189288909969212&elSBN 781305657571&snapshotld%=2199898&id%3.. * Tp * NDTAP Q Search this course Use the References to access important values if needed for this question. To determine the concentration of a solution of hydrochloric acid, a 125.0-mL sample is placed in a flask and titrated with a 0.1169 M solution of barium hydroxide. A volume of 40.55 mL is required to reach the phenolphthalein endpoint. Calculate the concentration of hydrochloric acid in the original sample. M Submit Answer 5 question attempts remainingarrow_forwardPls help ASAP on both questions.arrow_forward
- Use the following information to answer the next question. A student performed a number of qualitative tests on a sample of CsMn04 and recorded the following data. I. a resulting solution did conduct electricity II. the flame test of a resulting solution produced a violet colour III. a precipitate formed when LICIO4(aq) was added IV. a resulting solution was colourless The data that is inconsistent with expected results I II III IVarrow_forwardIf water was used to rinse the burette used to dispense the hydrochloric acid solution, how would this affect your final estimate of hydrochloric acid concentration? (More, less or unchanged?) Explain why, discussing the number of moles that you would actually have due to the rinsing.arrow_forwardSuppose you wanted to increase the buffer capacity of the formic acid/sodium formatebuffer solution above. What would you do? Explain your answer.arrow_forward
- Determine whether the stopcock should be completely open, partially open, or completely closed for each activity involved with titration. Filling the buret with titrant Choose... Close to the calculated endpoint of a titration Choose... Conditioning the buret with titrant Choose... At the beginning of a titration Choose...arrow_forward0.5 M of stock solution is given to be used for Titrant 1. Titrant 2 is going to be created by diluting 0.5 M stock solution to 0.1 M. How much stock solution (volume) will be needed to create 25 mL of the 0.1 M Titrant solution.arrow_forwardwhat is the balance equation wirh physical sates of butanoic acid and potassium butanoate as buffer solution.arrow_forward
- calculate to make up 1.0 M acetic acid from 80% concentrate.arrow_forwardSyl... A solution initial components B C Gr... D O ACIDS AND BASES Making qualitative estimates of pH change Each row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. . Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. . Use the fifth column to predict how the change in the solution will change its pH. H2O, KOH Explanation H₂O H2O, KOH We... H₂O Check O 14.... OO initial type (check all that apply) acidic basic neutral acidic basic neutral acidic basic neutral acidic basic neutral change add HClO4 add NaOH add K C104 add Nal 14.... X X effect of change on pH (check one) ООС 0 0 0/0 О С www-awu.aleks A ALEKS-... 27 pH higher pH lower pH the same pH higher pH lower pH the same pH higher pH lower OpH the same pH higher pH lower pH the same S n Ⓡ tv MacBook Aiarrow_forwardIf the amount of NaOH used in the titration against above HCI, was 26.7 ml, calculate the number of moles of NaOH at the end-point of the titration. (Give your answer to 2 significant figures)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY