
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the concentration?
what is shown in the pictures was all I was given
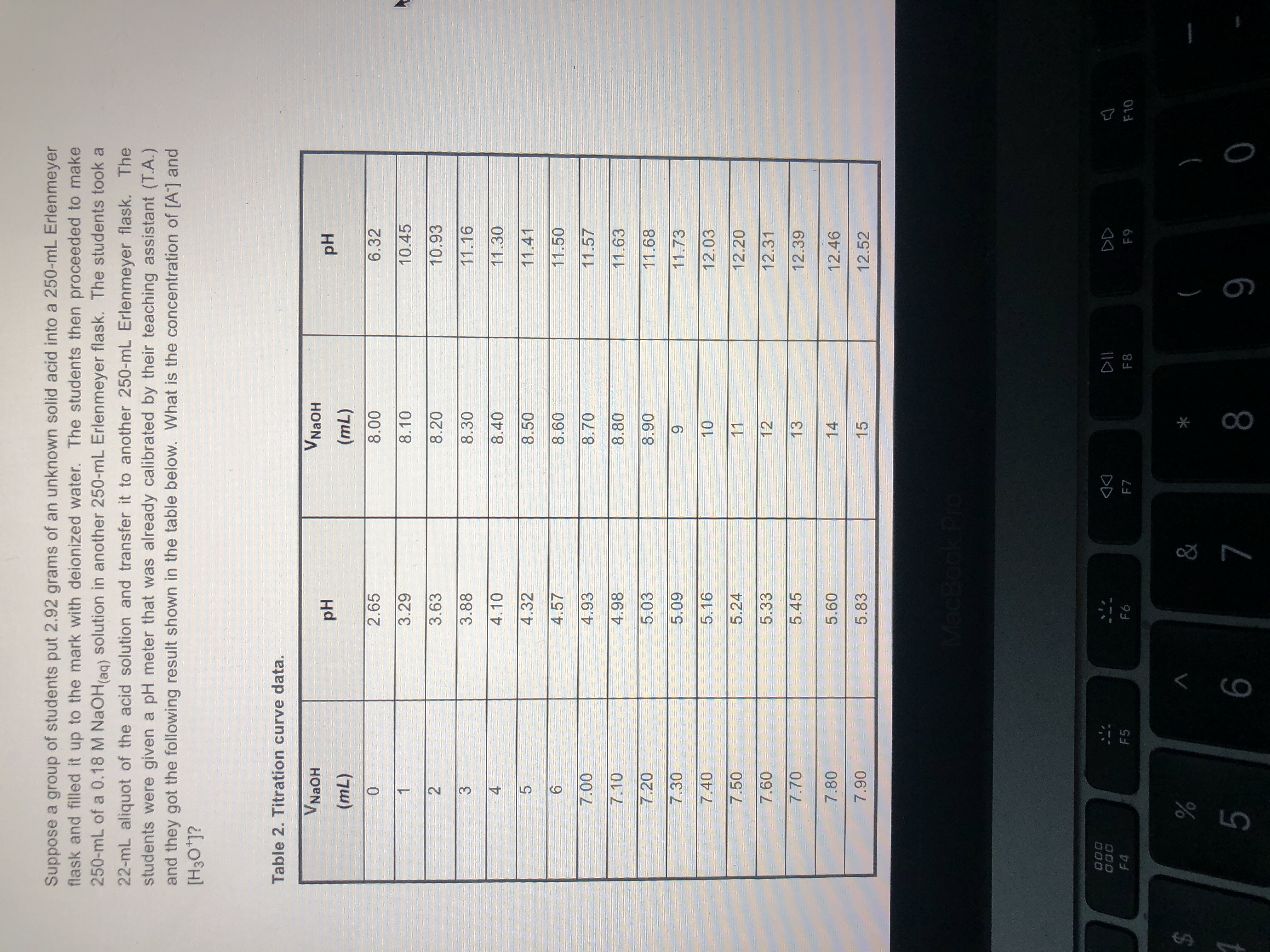
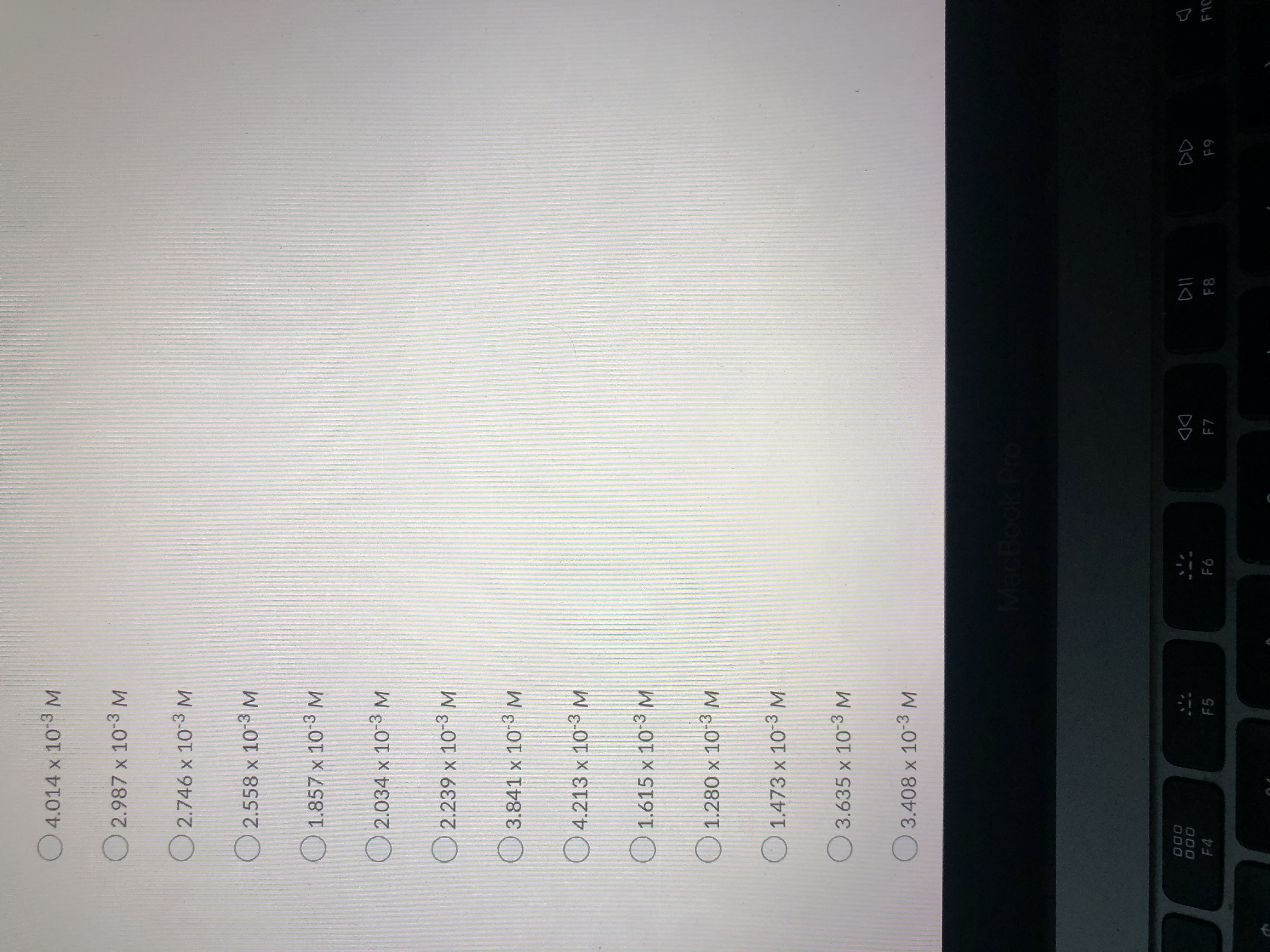
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of water is found to contain 0.012 ppm of Pb2* ions. Calculate the mass of lead ions per liter of this solution (assume the density of the water solution is 1.0 g/mL). O 5.9x10 g/L Pb2 O 1.2*10 g/L Pb O 1.2*10 11 g/L Pb2 O 1.2%10 5 g/L Pb2+ Activate Windows Go to Settings to activate Windo inspiron F10 F11 Backspac P F K Ente B Shift Alt Alt Ctrlarrow_forwardPlease answer question 1 Part A and Barrow_forward2. The slopes of the lines in graph 1 can be interpreted as a useful quantity around the lab if you were working with water or one of these solutions. Explain the significance of the slope and how it might be used around the lab. Hint: look at the units and remember when a line is drawn it represents a sort of average as it places about half of the data above and below it due to random errors.arrow_forward
- A solution has a concentration of 0.72M KBr and a volume of 0.020 L. It is then diluted to a concentration of 0.24 M. What is the new volume? Select the correct answer below: 0.060 L 0.0070 L 0.020 L 0.10 Larrow_forwardchemistry, Quicccckly please solve question 11arrow_forward39.6 g of carbon dioxide (CO2) is dissolved in a 2 L of water. 1 L of water is added. What is the new volume? What is the new molarity?arrow_forward
- A 340 L sample of seawater contains 8.90 g of gold(III) chloride. What is the concentration of gold(III) chloride in ppm? Express your answer with three significant digits. Do not include units! ppm What is the amount concentration (in mol/L) of sodium ions and carbonate ions when a 0.144 mol/L solution of sodium carbonate Express your answers with three significant digits. Do not include units! DELLarrow_forwardchemistry, Quicccckly please solve question 9arrow_forwardnitric acid could be used to neutralize barium hydroxide. if you have 0.432 L of a 0.555 M barium hydroxide solution, how much 3.00 M nitric acid will be required to neutralize it 0.240 L 0.160 L 0.0799 L or 0.719 Larrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY