
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the component concentration ratio, [Pr−]/[HPr], of a buffer that has a pH of 5.31?(Ka of HPr = 1.3 × 10−5)
Expert Solution
arrow_forward
Step 1
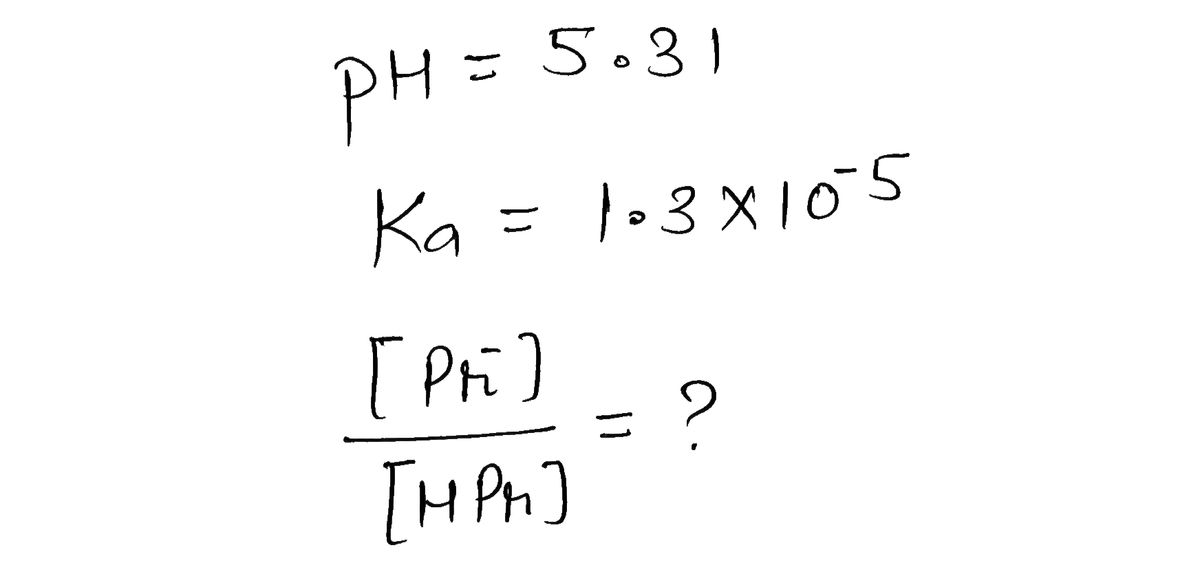
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What ratio of NaCN to HCN is needed to prepare a pH 10.10 buffer? (Ka of HCN is 4.9 × 1010)arrow_forwardHow many grams of sodium formate, HCOONa, are required to mix with 1.50 Liters of 0.500 M formic acid, HCOOH, in order to prepare a buffer solution with a pH that equals 4.00? pKa(HCOOH) = 3.74 and MM(HCOONa) = 52.01 g/molarrow_forwardWhich of the following aqueous mixtures will result in a buffer with a pH higher than 7.0? (For HCNO, Ka = 2.2×10-4, for NH3, Kb = 1.8×10-5 ) 10 mL of 0.1 M NH3 + 10 mL of 0.1 M HCl 10 mL of 0.1 M HCNO + 5.0 mL of 0.1 M NaOH 10 mL of 0.1 M HCNO + 10 mL of 0.1 M NaOH 10 mL of 0.1 M NH3 + 5.0 mL of 0.1 M HCl 10 mL of 0.1 M NH3 + 10 mL of 0.1 M HCNOarrow_forward
- What is the pH of the buffer that results when 2.3 g of NH3 and 6.46 g of NH4Cl are dissolved in 250 mL water? (Ka for NH4+ = 5.60 × 10-10) Please give the answer to 2 decimal places.arrow_forwardThe pKa of lactic acid is 3.86. What is the pH of a buffer in which the lactic acid concentration is 0.200 M and the sodium lactate concentration is 2.00 M?arrow_forwardConsider a buffer made by adding 132.8 g of NaC₇H₅O₂ to 300.0 mL of 2.35 M HC₇H₅O₂ (Ka = 6.3 x 10⁻⁵) a What is the pH of this buffer? b What is the pH of the buffer after 0.750 mol of H⁺ have been added? c What is the pH of the buffer after 0.250 mol of OH⁻ have been added?arrow_forward
- Which of the following aqueous mixtures will result in a buffer with a pH higher than 7.0? (For HCNO, Ka = 2.2×10-4, for NH3, Kb = 1.8×10-5 ) 10 mL of 0.1 M NH3 + 10 mL of 0.1 M HCl 10 mL of 0.1 M NH3 + 10 mL of 0.1 M HCNO 10 mL of 0.1 M NH3 + 5.0 mL of 0.1 M HCl (Correct answer) 10 mL of 0.1 M HCNO + 10 mL of 0.1 M NaOH 10 mL of 0.1 M HCNO + 5.0 mL of 0.1 M NaOH please explain. thanks!arrow_forwardWhat mass of sodium benzoate should you add to 158.0 mL of a 0.16 MM benzoic acid (HC7H5O2) solution to obtain a buffer with a pH of 4.29? ( Ka(HC7H5O2)=6.5×10−5)arrow_forwardWhat is the component concentration ratio, [Pr−]/[HPr], of a buffer that has a pH of 4.85?(Ka of HPr = 1.3 ×10−5)arrow_forward
- Calculate the molar ratio of NaFNaF to HFHF required to create a buffer with pH=4.20pH=4.20 . ( Ka(HF)=6.8×10−4Ka(HF)=6.8×10−4.)arrow_forwardEnter your answer in the provided box. What is the component concentration ratio, [Pr−]/[HPr], of a buffer that has a pH of 4.67? (Ka of HPr = 1.3 × 10−5)arrow_forwardWhat ratio of NaCN to HCN is needed to prepare a pH 10.00 buffer? (Ka of HCN is 4.9 × 10⁻¹⁰)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY