
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:**Question:**
What is the common name of the following compound?
**Diagram Explanation:**
The diagram depicts a structural formula of an organic compound. It consists of:
- A chain of three carbon atoms.
- The first carbon is bonded to a methyl group (CH₃).
- The second carbon is bonded to an oxygen atom, which is further bonded to another carbon.
This structure represents an ether, specifically with a branching methyl group on the second carbon and an ethyl group on the other side of the oxygen.
**Answer Box:**
A blank field is provided for the answer input.
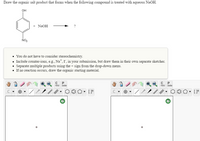
Transcribed Image Text:**Instruction for Educational Content:**
**Title: Reaction of Phenolic Compound with Aqueous NaOH**
**Objective:**
Draw the organic salt product that forms when the given phenolic compound is treated with aqueous NaOH.
**Chemical Reaction:**
- **Reactant:** A phenolic compound with a nitro group (NO₂) para to the hydroxyl group.
- **Reagent:** Aqueous NaOH
- **Product:** ?
**Guidelines:**
- Stereochemistry is not a consideration for this exercise.
- Remember to include counter-ions (e.g., Na⁺) in your drawn products, but they should be drawn in a separate sketcher.
- Use the plus sign to separate multiple products in your submission.
- If there is no reaction, redraw the organic starting material.
**Sketching Area:**
Two sketching panels are provided for drawing the structures. Utilize the toolbar to draw and modify chemical structures as needed.
**Note:** Ensure you save your work as it is entered in the chemical sketcher to avoid data loss.
**Additional Instructions:**
- Use available tools like the eraser, bond drawing, structure templates, and others provided in the toolbar to complete your drawing.
- Submit the final structure that accurately depicts the reaction product or the starting material if no reaction occurs.
Ensure clarity and accuracy in the depiction of chemical structures, as this aids in understanding and learning the nuances of chemical reactions involving phenolic compounds.
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY