
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
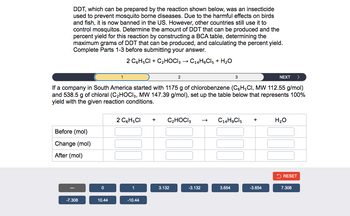
Transcribed Image Text:DDT, which can be prepared by the reaction shown below, was an insecticide
used to prevent mosquito borne diseases. Due to the harmful effects on birds
and fish, it is now banned in the US. However, other countries still use it to
control mosquitos. Determine the amount of DDT that can be produced and the
percent yield for this reaction by constructing a BCA table, determining the
maximum grams of DDT that can be produced, and calculating the percent yield.
Complete Parts 1-3 before submitting your answer.
2 C6H5Cl + C₂HOCI 3 → C14H9Cl5 + H₂O
Before (mol)
Change (mol)
After (mol)
-7.308
If a company in South America started with 1175 g of chlorobenzene (C6H5CI, MW 112.55 g/mol)
and 538.5 g of chloral (C₂HOCI3, MW 147.39 g/mol), set up the table below that represents 100%
yield with the given reaction conditions.
0
1
10.44
2 C6H5CI + C₂HOCI 3
1
2
-10.44
3.132
-3.132
C14H9Cl5 +
3.654
NEXT
-3.654
H₂O
RESET
7.308
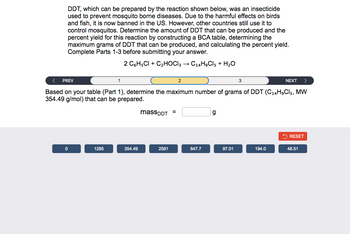
Transcribed Image Text:DDT, which can be prepared by the reaction shown below, was an insecticide
used to prevent mosquito borne diseases. Due to the harmful effects on birds
and fish, it is now banned in the US. However, other countries still use it to
control mosquitos. Determine the amount of DDT that can be produced and the
percent yield for this reaction by constructing a BCA table, determining the
maximum grams of DDT that can be produced, and calculating the percent yield.
Complete Parts 1-3 before submitting your answer.
2 C6H5Cl + C₂HOCI3 → C14H9Cl5 + H₂O
< PREV
NEXT >
Based on your table (Part 1), determine the maximum number of grams of DDT (C14H₂Cl5, MW
354.49 g/mol) that can be prepared.
0
1295
1
354.49
massDDT =
2591
2
647.7
g
97.01
3
194.0
RESET
48.51
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
What is the change in moles for h20
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
What is the change in moles for h20
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the % yield of the reaction? What is the limiting reagent? Show complete calculations for the theoretical and % yield calculations, please. Bromobenzene: 4.5mL Magnesium:1.0g Methyl benzoate:2.5mLarrow_forwardQuestion 25 of 97 Submit DDT was an insecticide used to prevent mosquito borne diseases. Due to the harmful effects on birds and fish, it is now banned in the US. However, other countries still use it to control mosquitos. It can be prepared by the reaction shown below. Determine the amount of DDT that can be produced and the percent yield for this reaction. 2C6H,CI + C2HOCI, – C,4H9CI, + H20 2 3 NEXT If a company in South America started with 1175 g of chlorobenzene (C6H5CI, MW 112.55 g/mol) and 538.5 g of chloral (C2HOCI3, MW 147.39 g/mol), set up the table below that represents 100% yield with the given reaction conditions. 2C6H,CI C2HOCI, C1.H,Cls H2O Before (mol) Change (mol) After (mol) O RESET 3.132 -3.132 3.654 -3.654 7.308 0. + -7.308 10.44 -10.44 MacBook Air F12 II F8 F10 F11 000 F9 20 F3 F7 F4 F5 F6 esc F2 F1 +.arrow_forwardAn excess of sodium carbonate, Na2CO3, in solution is added to a solution containing 17.87 g CaCl2. After performing the experiment, 12.51 g of calcium carbonate, CaCO3, is produced. Calculate the percent yield of this reaction.arrow_forward
- DDT, which can be prepared by the reaction shown below, was an insecticide used to prevent mosquito borne diseases. Due to the harmful effects on birds and fish, it is now banned in the US. However, other countries still use it to control mosquitos. Determine the amount of DDT that can be produced and the percent yield for this reaction by constructing a BCA table, determining the maximum grams of DDT that can be produced, and calculating the percent yield. Complete Parts 1-3 before submitting your answer. 1 Before (mol) Change (mol) After (mol) 2 C₂H₂CI+ C₂HOCI → C₁H₂Cl + H₂O 3 14 9 5 2 3 NEXT > If a company in South America started with 1175 g of chlorobenzene (CH₂CI, MW 112.55 g/mol) and 538.5 g of chloral (C₂HOCI, MW 147.39 g/mol), set up the table below that represents 100% yield with the given reaction conditions. 2 C₂H₂CI + C₂HOCI CH.CI 14 9 5 + H₂Oarrow_forwardFor the following reaction, determine the mass of Sulfur would be needed to obtain 80.0 g of CS2. The percent yield of the reaction is consistently 92%. CH4 (g) + 4S (g) → CS2 (g) + 2H2S (g)arrow_forward2. Ethyl chloride is prepared by the reaction of chlorine with ethane according to the following equation: C:Ho(g) + Cl2(g) – CH$CI(g) + HCl(g) Ethane Ethyl chloride a) Write the balanced chemical equation. Show proof of completion. b) When 5.6 g of ethane is reacted with excess chlorine, determine its theoretical yield c) Calculate the percent yield of ethyl chloride if experimental results indicated that 8.54 g of ethyl chloride had formed.arrow_forward
- If the theoretical yield of a reaction is 22.6 gg and the actual yield is 19.9 gg , what is the percent yield? Express your answer to three significant figures.arrow_forwardSodium nitrate, manganese (II) sulfate, potassium sulfate, and water is formed from the reaction of sodium nitrite, potassium permanganate, and sulfuric acid. Calculate the actual yield if a percentage yield of 33.01% of sodium nitrate is formed from 47.22 g sodium nitrite, 38.84 g potassium permanganate, and 42.88 g sulfuric acid. Atomic Mass: Na: 22.990 g/mol N: 14.007 g/mol O: 15.999 g/mol Mn: 54.938 g/mol H: 1.008 g/mol S: 32.065 g/mol K: 39.098 g/molarrow_forwardBalance and complete the following combustion reaction. Enter the sum of the balanced coefficients as your answer. Assign "blank" coefficients a value of 1. dicarbon herahydride (g) + oxygen (g) →?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY