
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
12.
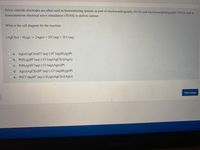
Transcribed Image Text:Silver chloride electrodes are often used as biomonitoring sensors as part of electrocardiography (ECG) and electroencephalography (EEG), and in
transcutaneous electrical nerve stimulation (TENS) to deliver current.
What is the cell diagram for the reaction:
2A£C1(s) + H2(g) → 2Ag(s) + 2H*(aq) + 2C1 (aq)
a. Ag(s)AgCl(s)|Cl (aq) || H*(aq)|H2(g)|Pt
b. Pt|H2(g)|H*(aq) || CI (aq)|AgCl(s)|Ag(s)
c. Pt|H2(g)|H*(aq) || CI (aq)|Ag(s)|Pt
O d. Ag(s)AGCI(s)|H*(aq) || Cl"(aq)|H2(g)|Pt
O e. Pt|Cl (aq)H*(aq) || H2(g)|AgCl(s)|Ag(s)
Next page
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When water and an electrolyte, such as salt, is added, the solution conducts electricity from a power source and water is decomposed. Where does the hydrogen gas form? Oat both ends O at the negative end O at neither end O at the positive end MacBook Air 80 D00 000 DD F7 F8 F9 F10 F3 F4 F5 F6arrow_forward(Q59) What is the molality of a solution that contains 73.7 grams of strontium fluoride that has been added to and dissolves in 3.13 liters of water? The density of water is 1.00 kg/L. (3 sf)arrow_forward(#26) Concentration of Solutions A concentrated solution of hydrochloric acid contains 120.0 g of water and 72.0 g of hydrochloric acid. (26a) What is the total moles of components in the final solution? Type-in your answer in Blank #1. Enter only the value in regular numerals with three significant figures. 26b) What is the mole fraction for hydrochloric acid? Type-in your answer in Blank #2. Enter only the value in regular numerals with no digit or space filler and with three significant figures. (26c) Given the density of the solution as 1.189 g/mL, what is the total volume of the solution? Type-in your answer in Blank #3. The unit is milliliters, but enter only the value in regular numerals with four significant figures (26d) What is the Molarity of the solution? Type-in your answer in Blank #4. The unit is mole/L but enter only the value in regular numerals with four significant figures. Dialarrow_forward
- Options for first blank: A. Exothermic B. Endothermic Options for second blank: A. Less B. More Options for third blank: A. Le chantelier’s principle B. Henry’s lawarrow_forward10:24 1 Done Introductory+Chemistry ill a 10. What mass of Li₂O is needed to react with 1,060 g of CO₂? Li₂O(aq) + CO₂(g) → Li₂CO3(aq),arrow_forwardI forgot how I got to this answer. Can you please show me how?arrow_forward
- 39arrow_forwardFor a molecular species, its molar mass in g/mole would be the same number as its molecular mass in amu when: A. the temperature is increased by 100 oC B. there are an Avogadro’s number of molecules C. there are two dozen molecules D. the molecular species is dissolved in a solvent to form a solution E. the temperature is 0 Karrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY