
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![**Question:**
What is the appropriate systematic name for the compound below?
[Image of chemical compound structure]
**Options:**
A. (1S,3R)-1-(1,1-dimethylethyl)-3-mercaptocyclohexane
B. (1R,3R)-3-tert-butylcyclohexanethiol
C. (1R,3S)-3-(1,1-dimethylethyl)cyclohexanethiol
D. cis-3-tert-butylcyclohexanethiol
**Compound Structure Explanation:**
The image presents a cyclohexane ring with two substituents. The substituents are positioned in such a way that one is an SH (thiol) group and the other is a tert-butyl group. The stereochemistry and exact positioning of these groups are critical to determining the compound's systematic name.](https://content.bartleby.com/qna-images/question/c0f85ff8-2ea0-4d43-a0c0-84de96cc63b8/6e7cf72b-a3ff-42f4-9f86-50bd877ed7b6/b3m4z2r_thumbnail.jpeg)
Transcribed Image Text:**Question:**
What is the appropriate systematic name for the compound below?
[Image of chemical compound structure]
**Options:**
A. (1S,3R)-1-(1,1-dimethylethyl)-3-mercaptocyclohexane
B. (1R,3R)-3-tert-butylcyclohexanethiol
C. (1R,3S)-3-(1,1-dimethylethyl)cyclohexanethiol
D. cis-3-tert-butylcyclohexanethiol
**Compound Structure Explanation:**
The image presents a cyclohexane ring with two substituents. The substituents are positioned in such a way that one is an SH (thiol) group and the other is a tert-butyl group. The stereochemistry and exact positioning of these groups are critical to determining the compound's systematic name.
Expert Solution
arrow_forward
Step 1
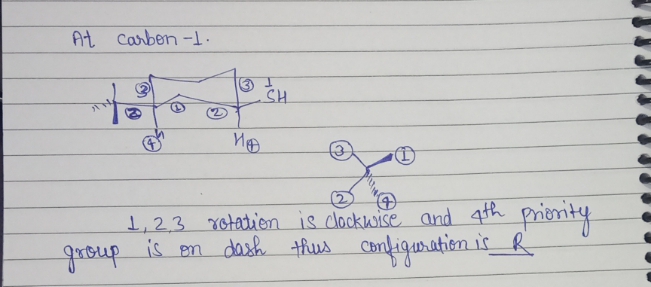
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Nonearrow_forwardWhat is an appropriate systematic name for the compound shown? (R)-2-methyl-5-(1-methylpropyl)cyclopentene O (R)-1-methyl-3-butylcyclopentene O (R)-1-methyl-3-(2-butyl)cyclopentene (R)-1-methyl-3-(1-methylpropyl)cyclopentenearrow_forwardWhich of the following will be obtained by the reduction of pentanal using NaBH4 followed by treatment with water? pentanoic acid 2-pentanol Opentene 1-pentanol Previous Page Next Page Page 5 of 24arrow_forward
- Rank the compounds in order of increasing stability (i.e. least stable to most stable). 02 < 02 < 022- <02²+ 022+ < 02 < 02 < 02²- 02 < 02 <022+ < 02- O 022- < 02 < 02 <022+arrow_forwardPart F Give the IUPAC name for the following compound: CH₂CH₂ CH₂CH₂CH, Spell out the IUPAC name of the compound. (1R,3S)-1-ethyl-3-propylcyclopenta Submit Previous Answers Request Answer X Incorrect; Try Againarrow_forwardB) Draw the correct structure for the product in each of the following mas Lad —CH—CH_* HH CC-CH₂-CH₂ +2H-H_ D) C) CH₂-CH₂-C-CH-CH₂-CH₂ +H-OH_ T afm CH₂ CH₂ H -CH+H-OH-arrow_forward
- r Provide the correct systematic name for the compound shown here. H3C Time's Up! 4- iso NH₂ CH3 (E)- (R)-(S)- (Z)- CH3 2- 6- 3- 5- sec- tri di cyclo tert- prop amino but meth nitro hex Submit +arrow_forwardDraw structures corresponding to the following names from 1 to 7.1 Draw structures corresponding to (2S,3R)-2,3,4-Trihydroxybutanal2 Draw structures corresponding to 2,2,4,4-Tetramethyl-3-pentanone3 Draw structures corresponding to 4-Methyl-3-penten-2-one4 Draw structures corresponding to Butanedial5 Draw structures corresponding to 3-Phenyl-2-propenal6 Draw structures corresponding to 6,6-Dimethyl-2,4-cyclohexadienone7 Draw structures corresponding to p-Nitroacetophenonearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY