
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
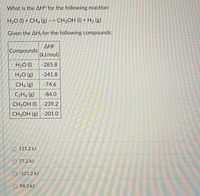
Transcribed Image Text:What is the AH° for the following reaction:
H20 (I) + CH4 (g) --> CH3OH (I) + H2 (g)
Given the AHf for the following compounds:
AHf
Compounds
(kJ/mol)
H2O (1)
-285.8
H2O (g)
-241.8
CH4 (g)
-74.6
C2H, (g)
-84.0
CH3OH (I) -239.2
CH3OH (g) -201.0
O 121.2 kJ
O 77.2 kJ
O -121.2 kJ
O 88.3 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the AH, information provided to calculate AH for the following: > SO,Cllg) + 2 H2O() AH, J/mol) 2 HCIG)+ H,S04) AH n 50,Cylg) -364 H,O -286 HCG) -92 H2SO -814 62 kJ 0-256 kJ 422 kJ O 256 kJ O -62 kJarrow_forwardH2 (g) + Cl2 (g) –→ 2 HCI (g) Using the following information, calculate AHrxn for the reaction above: NH3 (g) + HCI (g) → NH,CI (s) AHxn = -176 kJ/mol N2 (g) + 4 H2 (9) + Cl2 (g) → 2 NH,CI (s) AHxn = -628 kJ/mol | NH3 (g) → 1/2 N2 (g) + 3/2 H2 (g) AHxn= 46 kJ/mol number or fraction only kJ/molarrow_forwardCalculate the AH°rxn for the combustion of methane using the given AH°r. CH4 (g) + 202 (g) 2H20 (1) + CO2{g) AH°t, methane(g) = -74.60 kJ/mol AH°f, water(l) = -285.8 kJ/mol AH°t, carbon dioxide(g) = -393.5 kJ/mol 890.5 kJ/mol -890.5 kJ/mol -1.04 x 103 kJ/molarrow_forward
- Calculate AH for the reaction 2Sn(s) + O₂(g) → 2SnO(s) Given the following hypothetical data: Sn(s) + O₂(g) → SnO₂ (s) 2SnO(s) + O₂(g) → 2SnO₂ (s) ΔΗ AH = KJ AH AH-581 kJ - 2SnO₂ (s) AH = − 592 kJarrow_forwardGiven the following information: Pb(s) + PbO2(s) + 2H2SO4(l) ---> 2PbSO4(s) + 2H2O (l) DHrxn = 509.2 KJ/mol SO3 (g) + H2O (l) ---> H2SO4 (l) DHoRxn = -130.3KJ/mol Determine DHoRxn for: Pb (s) + PbO2(s) + 2SO3 (g) ----> 2PbSO4 (s)arrow_forwardreaction W Calculate AG rxn For the following at 450k CH ₂0 (g) + 2H₂ (9) → CHH (g) + H₂O (9) Using AH = -94.9 KJ; A'S = -224.2J/Karrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY