
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
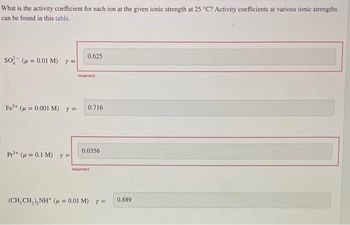
Transcribed Image Text:What is the activity coefficient for each ion at the given ionic strength at 25 °C? Activity coefficients at various ionic strengths
can be found in this table.
SO³- ( = 0.01 M) y =
0.625
Pr¹+ (μ = 0.1 M) y =
Incorrect
Fe³+ (μ = 0.001 M) y = 0.716
0.0356
Incorrect
(CH,CH,),NH* ( =0,01M) y=
0.889
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- need help on these two please A generic salt, AB, has a molar mass of 351 g/mol and a solubility of 1.50 g/L at 25 °C. AB(s) = A*(aq) +B (aq) What is the Kp of this salt at 25 °C? Kp = 4.27 x10-3 Incorrect The formation constant of (M(CN), is 2.50 x 10", where M is a generic metal. A 0.160 mole quantity of M(NO,); is added to a liter of 1.320 M NaCN solution. What is the concentration of M2 ions at equilibrium? (M2) =arrow_forwardFor the aqueous Hg(NH,), complex K, = 1.8 × 101º at 25 °C. K; =1 Suppose equal volumes of 0.0010M Hg(NO,), solution and 0.20M NH, solution are mixed. Calculate the equilibrium molarity of aqueous Hg“ ion. 2 Round your answer to 2 significant digits. M ?arrow_forwardsecond questionarrow_forward
- Calculate the pOH of a 0.0376 M HCIO solution at each temperature. 0°C pOH = 35 °C pOH = T (°C) 0 5 10 15 20 30 35 40 45 50 100 Kw 1.15 x 10 1.88 x 10 2.97 x 10 4.57 x 10 6.88 x 10 1.46 × 10 2.07 x 10 2.88 x 10 3.94 x 10 5.31 x 10 5.43 x 10 45 45 44 H 45 15 15 14 +4 44arrow_forwardGiven the equilibrium reaction MgF2(s) ↔ Mg+2(aq) + 2F-(aq) Ksp = 7.4x10-11 Calculate the amount of MgF2(s) in grams needed to create One Liter of a saturated solution in standard conditions?arrow_forwardYou are asked to weigh 2 g of lithium and add it to 1500 ml of distilled water +2 drops of indicator, knowing that the density of water is 1 at room temperature while conducting this experiment. After determining the molarity of the LIOH, you need to know how many volume should be taken to reach the final concentration. Therefore, use the one underneath, so the molarity after dilution will be 0.025 M instead of 0.25 MAfter knowing the amount of LiOH, you need to dilute to prepare 0.025 M of LiOH in a 3000 ml solution. Show your calculation and units.arrow_forward
- What is the activity coefficient for each ion at the given ionic strength at 25 °C? Activity coefficients at various ionic strengths can be found in this table. SO (μ= 0.1 M) y = Y³+ (μ = 0.01 M) y = Pr³3 + (μ = 0.1 M) Y = (CH₂CH₂)₂NH+ (μ = 0.05 M) y = 0.8 x10 TOOLSarrow_forwardThe formation constant* of [M(CN) 2 ]^ - is 5.30 * 10 ^ 18 , where M is a generic metal. A 0.140 mole quantity of M(NO 3 ) is added to a liter of 0.750 M solution. What is the concentration of mathcal W ^ + at equilibrium?[M^+2]=arrow_forwardCalculate eq. concentrations when for 0.55 M HAc solution where ka(25C)=1.8x10^-5 with an ICE table. Calculate the pH of solution and percent ionization. Why does this pH make sense compared to a strong acid such as hydrochloric acid?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY