
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
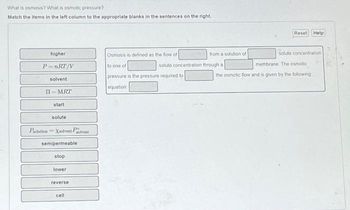
Transcribed Image Text:What is osmosis? What is osmotic pressure?
Match the items in the left column to the appropriate blanks in the sentences on the right.
higher
P=nRT/V
solvent
II-MRT
start
solute
Psolation Xsolvent Polvent
semipermeable
stop
lower
reverse
cell
Osmosis is defined as the flow of
solute concentration
to one of
pressure is the pressure required to
equation
from a solution of
Reset Help
through a
solute concentration.
membrane. The osmotic
the osmotic flow and is given by the following
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- 1. The CaCO3 slurry is to be filtered in a filter press having 0.05 m2 area at a constant pressure of 400 kPa. The filtration equation was found to be; = (8 × 106) + 5000 V t; sec, V; m³, -AP=kPa Calculate the time needed to get 10 m3 filtrate for the same slurry and filter material, if the area of filter is 2 m? at a constant pressure of 200 kPa. (The specific resistance of filter is independent of pressure)arrow_forwardA stream containing propionic acid and water at 60:40 mass ratio is fed to a mixer at 3 kg/s with pure cyclohexane as solvent. What should be the flow rate of the solvent in order to attain 40:60 mass ratio of solute and solvent in the extract (solvent-rich) stream? Prepare graphical and computational solution.arrow_forwardWhen the pump in the Figure below draws 220 m³/hr of water at 20°C (p = 998 kg/m³) from the reservoir, the total friction head loss is 5 m. The flow discharges through a nozzle to the atmosphere. Estimate the pump power in kW delivered to the water. Water 2m 6 m D = 12 cm Pump De=5 cmarrow_forward
- In a membrane filtration system consisting of two membrane modules A, and B. if the pressure at feed side of A is 60 psi and permeate side of A is 30 psi. What will happen to the pressure of feed size after filtering water for about 6 months? O Pressure at feed side of A will increase O Pressure at feed side of A will decrease O Pressure at permeate side of A will increase 4arrow_forward1. Sodium chloride is used as a tracer to determine the probe to measure concențrations water injection dispersion coefficient in an aquifer. A 0.010 molar injection monitoring well #1 well #2 ground surface sodium chloride solution is injected into the aquifer at well #1 at a rate of 1 m/min. The injected water flows impermeable clay boundary radially outward from the well, only in the horizontal 10|m direction, and the only flow in the aquifer is due to the injected salt solution. The aquifer is 10 m thick and has a porosity of 0.35. impermeable clay boundary a) Write the PDE that describes the salt concentration at observation well #2, which is 10 m from the injection well. Include all initial and boundary conditions. b) Write the PDE, including all initial and boundary conditions, for injection of a 0.010 molar solution of dC = -k C methanol, which biodegrades according to: dt c) What physical processes contribute to dispersion in this problem? d) What is the steady state solution…arrow_forwardBlock flow chart required to produce liquid sorbitol from wheatarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The