
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
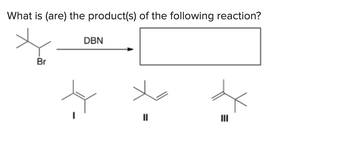
Transcribed Image Text:What is (are) the product(s) of the following reaction?
Br
DBN
11
III
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If exactly 4.089 moles of propanol (C3H8O) is combusted in the presence of excess oxygen what is the mass of O2 used in the reaction? The molecular weights of all reactants and products are: C3H8O: 60.09 g/mol O2: 31.998 g/mol H2O: 18.016 g/mol; CO2 : 44.01 g/mol. Enter your answer in the units of g, do not enter the unit itself.arrow_forwardDetermine the physical states for the products and then determine which factor does NOT drive reactions (i) and (ii) to product formation? 1) 2KO2(s) + 2H2O(1) → 2KOH(?)+H2O(1) + O2(?) (ii) 4KO2(s) + 2CO2(g)→ 2KCO3(?)+302(?) Select one: O A. For (i), formation of a water O B. For (ii), formation of O2 O C. For (ii), formation of K2CO3 O D. For (i), formation of a gas O E. For (i) and (ii), generation of a more stable oxidation state for Oarrow_forwardProvide the major expected products: MgBr 1. CO2(s) 2. HCI, H2Oarrow_forward
- For the reaction shown, which of the following answers best describes the product(s) of this reaction? Choose one answer OH م Br, OH Br₂ H₂O 25 °C Br HO Br b) Br, "11 OH Br Br + HO Br Br Brarrow_forwardPredict the products and what is the reaction mechanism?arrow_forwardPlease answer the following question: Why is the following reaction important in our everyday lives? 2NaN3 (s) → 2Na (s) + 3N2 (g)arrow_forward
- What is the classification of the following reaction: CaSO4 + Mg --> MgSO4 + Ca Single replacement combustion double replacement decompositionarrow_forwardWhich of the following is a product of the reaction of K2CO3(aq) with Zn(NO3)2 (aq)? Aqueous K2NO3 O Aqueous Zn(Co2 Solid ZnCO3 Solid KNO3arrow_forwardWhich are the reactants in this reaction? 6 CO2 + 6 H2O ---> C6H12O6 + 6O2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY