
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Ll.46.
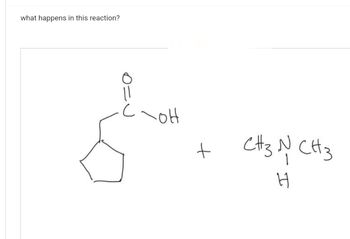
Transcribed Image Text:what happens in this reaction?
210
с
LOH
+ CHz Ņ CH3
N
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You were tasked to separate the components of a mixture containing silica, sodium chloride and charcoal. TNāCI dissolves in water while silica and charcoal are not water-soluble. Only charcoal dissolves in carbon disulfide. a. Write a short experimental procedure to carry out the separation of the mixture. b. Given the following data, determine the percentage of charcoal, sodium chloride and silica. Mass (g) Mass of beaker 100.000 Mass of beaker + mixture 110.000 mass of evaporating dish 62.000 mass of evaporating dish + solid after evaporation of water 65.000 Mass of beaker + charcoal + silica after evaporation of excess water 117.000 mass of beaker + silica after decanting dissolved charcoal and drying 113.545arrow_forwardCI 7.arrow_forwardDetermine the concentrations of hydronium and hydroxide ions in 0.076733152673 M aqueous sodium hydroxide.Kw = 1.0E-14. a. Hydroxide ion concentration? b. Hydronium ion concentration?arrow_forward
- chemistryarrow_forward1. Which of the following is not a step in preparing a water sample container?a. All sample containers must be dark in colorb. The type of sample container and the level of cleaning required depend on the type of sample to be takenc. All sample containers must be thoroughly cleaned in the laboratory before sampling is carried outd. The number of containers prepared must always be in excess of what is needed, for quality assurance, quality control and reserves 2. The purpose of environmental sample analysis is..a. To determine the origin and concentration of chemicals in the environmentb. To determine the origin, concentration of chemicals and/or pollutants in the environmentc. To determine the concentration of a chemical in the environmentd. To determine the cause and concentration of pollutants in the environmentarrow_forwardEtO NaOEt, ELOH EtO,C- OEt a) b.) CI EtO HO ČI ÓEt ÓH d.) e)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY