
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
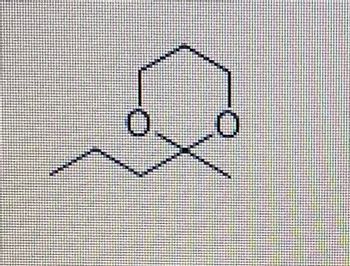
What carbonyl compound would be used to make the ketal shown here?
2-butanone
2-pentanone
1,2-propanedial
Butanal
Transcribed Image Text:0
0
Expert Solution
arrow_forward
Step 1 Introduction
- It is an protection of carbonyl group.
- The carbonyl group is proteced with using 1,2-diol.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardHello, If you could please help me solve this problem. These are the options for each reagent. 1: HCl/H2OHCl/H2O CH3NH2CH3NH2 NaOH/H2ONaOH/H2O methanol 2: NaOH/H2O CH3NH2 methanol HCl/H2O 5. NaOH/H2O CH3NH2 methanol HCl/H2O 6 NaOH/H2O CH3NH2 methanol HCl/H2Oarrow_forwardPlease show out how we might synthesize each of the following compounds from 1-butanolarrow_forward
- please make sure the first one is the COMMON namearrow_forward1-Ethoxypropane is: O CH3CH₂OCH₂CH³ Ⓒ CH³CHCH₂OCH3 O CH3CH₂CH₂CH₂CH3 O CH3CH₂CH2CH2CH₂CH3 OCH3CH₂CH₂CH₂OCH3arrow_forwardWhat best describes what happens to side A and B when the labeled molecule below it is refluxed in aqueous HI? Side A forms an alkyl iodide and Side B forms a ketone. O Side A forms an alkyl iodide and Side B forms an alcohol. O Side A forms an alcohol and Side B forms an alkyl iodide. O Side A forms a ketone and Side B forms an alkyl iodide. Side A 0 Side B 4arrow_forward
- How to synthesise 1-cyclohexylcyclohexanol from cyclohexanone?arrow_forwardX Your answer is incorrect. Try again. Provide the IUPAC name for the following compound:arrow_forwardFor each compound predict if it's positive or negative for AgNO3/EtOH Test (SN1), propose 1 sentence explanation why. 2-Bromobutane 1-bromobutane 2-Chlorobutane 1-chlorobutane t-butyl chloride benzyl chloride bromobenzene bromocyclohexane bromocyclopentanearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY