
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
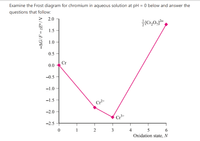
Transcribed Image Text:Examine the Frost diagram for chromium in aqueous solution at pH = 0 below and answer the
questions that follow:
2.0
Cr,0,-,
1.5
1.0 -
0.5
Cr
0.0
-0.5
-1.0
-1.5
Cr2+
-2.0
Cr*
-2.5
1
3
4
Oxidation state, N
A/ o7Z = /DV-

Transcribed Image Text:What can you say about the values of the standard reduction potential of each species in
the Frost diagram?
What can you say about the dichromate species (Cr2O,²-) from the information given to
you in the Frost diagram?
Which species is the most thermodynamically stable? Give a reason for your answer.
Is there evidence of possible disproportionation for chromium? Why or why not?
What can you say about the chromium species (Cr²*) from the information given to you in
the Frost diagram?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For (a.) Al3+|Al, (b.) Cu2+|Cu, (c.) Li+|Li, (d.) Cd2+|Cd, (e.) Au3+|Au Given redox couples, determine the temperature at which the redox couples are in equilibrium considering under standard conditions: 25 °C 1 M concentration for each ion participating in the reaction The partial pressure of 1 atm for each gas that is part of the reactionarrow_forwardExplain why copper(II) and iodide ions do not co-exist in acidic solution but do co-exist in the presence of ammonia. I want solution ASAParrow_forwardComplete and balance the following half-reaction in acidic solution TiO₂ (s) → Ti²+ (aq) + O₂²(aq)arrow_forward
- Write a balanced half-reaction describing the reduction of aqueous chromium(IV) cations to aqueous chromium(II) cations.arrow_forwardIf a 6.0 Amp current is used, calculate the number of hours needed to prepare 22.0 grams of calcium metal from calcium ion. The reduction potential is -2.39 Varrow_forwardWhat is linkage isomerisation and why can it be important?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY