
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
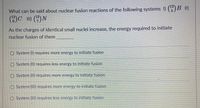
Transcribed Image Text:What can be said about nuclear fusion reactions of the following systems I) (5)B I)
12
6.
As the charges of identical small nuclei increase, the energy required to initiate
nuclear fusion of them
System (I) requires more energy to initiate fusion
O System (II) requires less energy to initiate fusion
O System (II) requires more energy to initiate fusion
O System (Ill) requires more energy to initiate fusion
O System (III) requires less energy to initiate fusion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 1 What mass of fissionable material is desired in a nuclear reactor? O A critical mass A decay mass A supercritical mass A subcritical mass « Previousarrow_forwardPlease see attached question.arrow_forwardWrite a natural occurring chemical reaction for nuclear fission, and one for nuclear fusion. (Elements must be in their natural form)arrow_forward
- A nuclear power station delivers 1 GW of electricity for a year from uranium fission. Giventhat a single fission event delivers about 200 MeV of heat, estimate the number of atomsthat underwent fission, their mass, and the loss of mass of the fuel elements.arrow_forwardPRACTICE QUESTIONS 42 of 48 Radioisotopes are used in computer screens in galvanic cells as tracers in scientific studies in rechargeable batteries Question 43 of 48 What is the nuclear binding energy for a mole of beryllium (Be-9), whose mass is 9.01218? (mass of a proton = 1.00728 AMU; neutron = 1.00866) 5.41x1015J 5.41x1012J 8.11x1017J 8.11x1014Jarrow_forwardNuclear fission and nuclear fusion each release enormous amounts of energy as a result of changes in the nuclei of atoms. Distinguish be- tween fission and fusion by comparing the process, the starting mate- rial, and the location where each commonly occurs. Answer in complete sentences.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY