Question
What are the major products of these reactions? Please show the mechanisms.
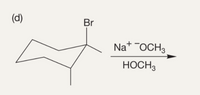
Transcribed Image Text:The image displays a chemical reaction involving an organic compound structure. The structure is a bicyclic compound with a bromine (Br) substituent. The reaction depicted involves sodium methoxide (Na⁺OCH₃⁻) in methanol (HOCH₃) as the reagent.
1. **Starting Material:**
- The compound shown is a bicyclic structure with alkyl substituents and a bromine atom attached.
2. **Reaction Conditions:**
- Reagent: Sodium methoxide (Na⁺OCH₃⁻)
- Solvent: Methanol (HOCH₃)
3. **Reaction Process:**
- The sodium methoxide acts as a base/nucleophile. This setup suggests the possibility of a nucleophilic substitution or an elimination reaction, typical for alkyl halides like the bromo compound in this structure.
Understanding such reactions is crucial in organic chemistry, particularly in the study of reaction mechanisms and the synthesis of complex organic molecules.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images
