
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
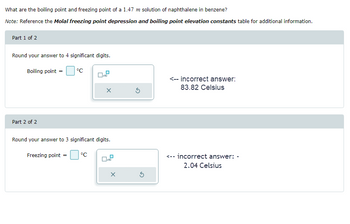
Transcribed Image Text:What are the boiling point and freezing point of a 1.47 m solution of naphthalene in benzene?
Note: Reference the Molal freezing point depression and boiling point elevation constants table for additional information.
Part 1 of 2
Round your answer to 4 significant digits.
Boiling point =
Part 2 of 2
°C
X
Round your answer to 3 significant digits.
Freezing point =
°C
X
Ś
<-- incorrect answer:
83.82 Celsius
<-- incorrect answer: -
2.04 Celsius
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A certain liquid X has a normal freezing point of -6.20 °C and a freezing point depression constant K, = 5.32 °C kg-mol. Calculate the freezing point of a solution made of 3.7g of sodium chloride (NaCl) dissolved in 100. g of X. Round your answer to 3 significant digits. 0°C X 0 Aarrow_forward8. A certain substance X has a normal freezing point of -8.4 °C and a molal freezing point depression constant K, = 2.33 °C·kg-mol Calculate the freezing point of a solution made of 63.48 g of urea ((NH2),CO) dissolved in 700. g of X. Round your answer to 3 significant digits. 0c Submit Assignment Continue O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility II Finder F11 F12 F9 F10 F8arrow_forwardPayalarrow_forward
- Calculate the boiling point of a solution of 400 g of ethylene glycol dissolved in 400 g of water Kf= 1.86 Celsius/m and KB = 0.512 Celsius/m. Use 100 degrees Celsius as the boiling point of waterarrow_forward* 00 LL LI #3 The normal freezing point of a certain liquid X is -2.70 °C, but when 43.3 g of potassium bromide (KBr) are dissolved in 400. g of X the solution freezes at -9.6 °C instead. Use this information to calculate the molal freezing point depression constant K, of X. Round your answer to 2 significant digits. °C-kg K. mol Check Save For Later Submit Assignme O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibil MacBook Pro 114 F8 F3 F4 $4 3. 5. 6. P. A G H. K. B alt alt option command command optionarrow_forwardKindly provide a CLEAR and COMPLETE solution. Please answer ASAP, thanks.arrow_forward
- Calculate the boiling point (in °C of a solution that is made from 48.6 g of a nonelectrolyte (M = 174.0785 g/mol) dissolved in 186.3 g of solvent. The pure solvent boils at 81.34 °C and its K, value is 3.97 °C/m. Report your answer to TWO places past the decimal.arrow_forwardCalculate the quantity (mL) of glycerin needed to prepare 250 mL of the following ophthalmic solution. Report your answer to 2 decimal places. DO NOT include units. Erythromycin lactobionate Dexamethasone sodium phosphate Glycerin Sterile water for injection, ad 500 mg 100 mg 2.5 mL 100 mLarrow_forwardWhat is the freezing point of a solution prepared by dissolving 14.270 g calcium nitrate in 108.800 g of water? The answer is not shown. -1.22 degrees Celcius -1.49 degrees Celcius -4.46 degrees Celcius 98.51 degrees Celciusarrow_forward
- What are the boiling point and freezing point of a 1.47 m solution of naphthalene in benzene? Note: Reference the Molal freezing point depression and boiling point elevation constants table for additional information. Part 1 of 2 Round your answer to 4 significant digits. Boiling point = Part 2 of 2 °C X Round your answer to 3 significant digits. Freezing point = X 3arrow_forwardA certain liquid X has a normal freezing point of 1.30 °C and a freezing point depression constant K,= 7.47 °C·kg•mol . Calculate the freezing point of a -1 solution made of 5.61g of alanine (C,H,NO,) dissolved in 300. g of X. Round your answer to 2 significant digits. °c x10 Continue Submit Assignment O2021 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility MacBook Air DII DD F7 F8 F9 F10 F11 00arrow_forwardUrea (NH2)2CO, is dissolved in 105.0 g of water. The solution freezes at -0.605°C. How many grams of urea were dissolved to make this solution? The Kf=1.858°C/m for water and its freezing point is 0.000°C. Express your answer to two decimal places.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY