
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%

Transcribed Image Text:* What amount (in mol) and what mass (in kg) of carbon dioxide are produced when 20 gallons
(approximately 2.0 x 10' kg) of octane (C3H18) is combusted?
C3H18(1) +O2(g) CO2(g) + H;O(1)
A: 1.4 x 10°; 62
Expert Solution
arrow_forward
Step 1 - Balanced Reaction for combustion
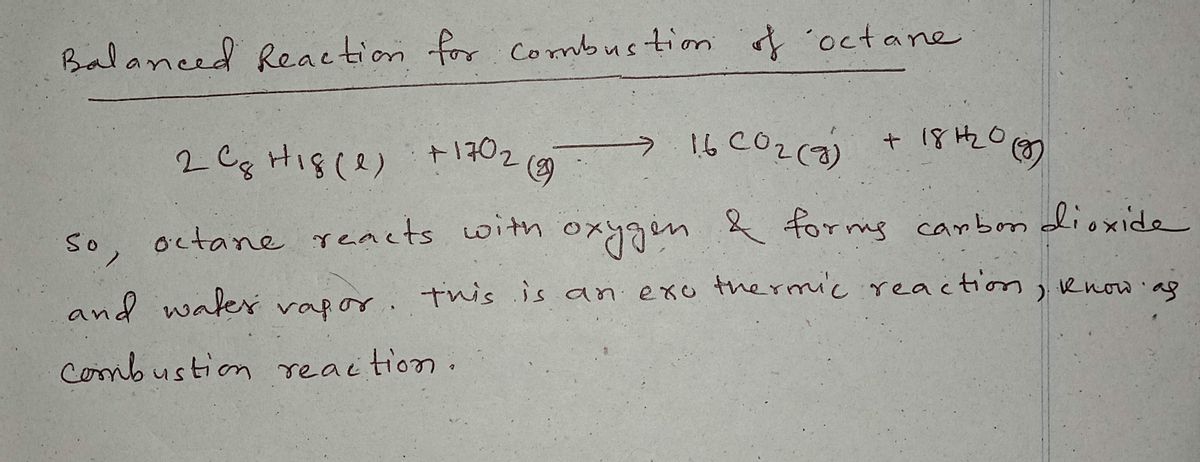
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Tungsten metal, used in light bulb filaments, is produced from the reaction between tungsten (VI) oxide and hydrogen: WO; (s) + 3 H:(g)_ → W (s) + 3 H;O () Calculate the mass of tungsten metal (in kg) that can actually be produced from 34.5 kg of tungsten (VI) oxide if the percent yield of the reaction is 75%.arrow_forwardID the molecules( XeF2O, CIO4, HNO2, CH3CH(NH2)COOH) with the following labels (1 is more than one label): Oxyacid, has a resonance structure, expanded octet, has a carbon backbone.arrow_forwardFill in blank.arrow_forward
- Im havin g toublr understanding what I did wrong on this conversion, can someone write it out and walk through it like in the question?arrow_forwardConsider the reaction involving the burning of sulfur: 2S(s) + 3O2(g) ->> 2SO3(g) delta Hrxn= ? ? ?arrow_forwardConsider the reaction. heat 2 Al(s) + Fe,03(s) Al,0;(s) + 2 Fe(l) If 11.5 kg Al reacts with an excess of Fe,O3, how many kilograms of Al,O, will be produced? mass of Al,0, produced:arrow_forward
- Balance the following chemical equation (if necessary):arrow_forwardHydrogen is manufactured on an industrial scale by this sequence of reactions: CH, (g) + H,0 (g) CO (g)+3 H, (g) K1 CO (g) + H,0 (g)= CO, (g)+H, (g) K2 The net reaction is: CH4 (g) +2 H,0 (g) CO, (g)+4H, (g) K Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K, and K,. If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator. Karrow_forwardE-85 is an alternative fuel for automobiles and light trucks that consists of 85.0% (by volume) ethanol (C2H5OH), and 15.0% gasoline. When ethanol burns completely it produces CO2 and H2O. The balanced equation for the burning of ethanol isC2H5OH+3O2-->2CO2+3H2OThe density of ethanol is 0.790 g/mL. How many moles of carbon dioxide are produced by the complete combustion of the quantity of ethanol in 8.00 gallons of E-85 fuel? _mol CO2arrow_forward
- A household water heater containing 50 gallons (227 L) of water is heated burning natural gas (methane, molar mass = 16.043 g mol-1) via the following reaction: CH4(g) + O2(g) CO2(g) + H20(O A,H = -891 kJ mol-1 What mass of methane, in units of g, is needed to heat the 227 L of water in the heater from 25.0°C to 44.0°C? Assume: • the heater is 100% efficient in its use of heat energy • no heat is lost to the surroundings specific heat of water is: 4.184 Jg-1 °C-1 density of water is: 0.9970 g mL¯1arrow_forwardA 1.50 g sample of a compound containing only C,H and O was burned completely. The only combustion products were 1.738 g CO2 and 0.711 g of water. What is the empirical formula and molecular formula of the compound? (MW = 152g/mol)arrow_forward00 %24 4+ dl w/ S' Enter your answer in the provided box. Using the balanced equation for the combustion of ethanol, answer the following question. C2H,O(1) + 3 02(g) – 2 CO2(g) + 3 H,0 (g) ethanol How many grams of H,0 are formed from 1.2 mol of ethanol? OH 8 #3 5. 9. 2 4 0Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY