
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Ok what about this one? I will also put the answer I got in there so you can check if I'm correct or not, or if I need to write it another way.
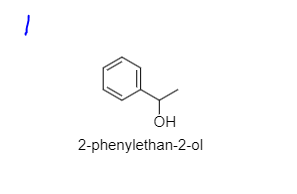
Transcribed Image Text:### Chemical Structure of 2-phenylethan-2-ol
#### Description:
The image above depicts the chemical structure of 2-phenylethan-2-ol, which is a type of alcohol.
#### Structural Details:
- **Benzene Ring**: The left part of the structure is a benzene ring, which consists of six carbon atoms arranged in a hexagonal shape with alternating double bonds.
- **Hydroxyl Group (OH)**: Attached to the second carbon atom in the ethyl chain from the benzene ring is a hydroxyl group, represented by "OH". This defines the compound as an alcohol.
- **Ethyl Chain**: The linear ethyl chain is connected to the benzene ring and contains the hydroxyl group.
#### Nomenclature:
The compound is named "2-phenylethan-2-ol," indicating a two-carbon chain (ethan) with an alcohol group at the second carbon position, and a phenyl group (benzene ring) attached to the first carbon.
This compound is known for its fragrance and is used in various perfumes and flavorings.
![**Spectral Analysis of a Pure Compound**
**Information:**
Each spectrum below was obtained from a pure compound.
- *Mass Spectrum*: Parent peaks (M) are listed for all examples.
- *Infrared (IR) Spectroscopy*: Peaks listed are strong (s) unless otherwise noted for signals above 1500 cm⁻¹.
- *¹H NMR Spectra*: The integral is given in the number of hydrogens (1H) or as a relative ratio. Important coupling constants (J-values) are indicated next to the peaks for some examples. For specific spectra, an inset (grey box) shows a “zoom-in” on a key part of the spectrum.
**Mass Spectrometry (not shown):** [M] = 122 m/z
**Infrared Spectroscopy (not shown):** 3364, 3030, 2973, 1493, 1451, 1408, 1178 cm⁻¹
**¹H Nuclear Magnetic Resonance:**
- A spectrum with a range from 0 to 8 PPM.
- Peaks are labeled as follows:
- At approximately 7.1 PPM, labeled "m" corresponding to 5H.
- At approximately 4.1 PPM, labeled "q" corresponding to 1H.
- At approximately 3.5 PPM, labeled "s" corresponding to 1H.
- At approximately 1.3 PPM, labeled "d" corresponding to 3H.
**¹³C Nuclear Magnetic Resonance:**
- A spectrum with a range from 0 to 160 PPM. It contains several distinct peaks.
This educational page provides insights into how various spectral data can be used to study pure compounds, offering foundational knowledge in structural analysis techniques such as Mass Spectrometry, Infrared Spectroscopy, and Nuclear Magnetic Resonance (NMR).](https://content.bartleby.com/qna-images/question/b5f1630e-c465-44f2-a868-b429d99d07e1/35fc05f6-0da3-4d10-99e1-a424580a5c56/d409hp_thumbnail.png)
Transcribed Image Text:**Spectral Analysis of a Pure Compound**
**Information:**
Each spectrum below was obtained from a pure compound.
- *Mass Spectrum*: Parent peaks (M) are listed for all examples.
- *Infrared (IR) Spectroscopy*: Peaks listed are strong (s) unless otherwise noted for signals above 1500 cm⁻¹.
- *¹H NMR Spectra*: The integral is given in the number of hydrogens (1H) or as a relative ratio. Important coupling constants (J-values) are indicated next to the peaks for some examples. For specific spectra, an inset (grey box) shows a “zoom-in” on a key part of the spectrum.
**Mass Spectrometry (not shown):** [M] = 122 m/z
**Infrared Spectroscopy (not shown):** 3364, 3030, 2973, 1493, 1451, 1408, 1178 cm⁻¹
**¹H Nuclear Magnetic Resonance:**
- A spectrum with a range from 0 to 8 PPM.
- Peaks are labeled as follows:
- At approximately 7.1 PPM, labeled "m" corresponding to 5H.
- At approximately 4.1 PPM, labeled "q" corresponding to 1H.
- At approximately 3.5 PPM, labeled "s" corresponding to 1H.
- At approximately 1.3 PPM, labeled "d" corresponding to 3H.
**¹³C Nuclear Magnetic Resonance:**
- A spectrum with a range from 0 to 160 PPM. It contains several distinct peaks.
This educational page provides insights into how various spectral data can be used to study pure compounds, offering foundational knowledge in structural analysis techniques such as Mass Spectrometry, Infrared Spectroscopy, and Nuclear Magnetic Resonance (NMR).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
what about this?
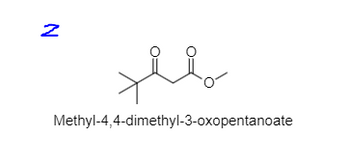
Transcribed Image Text:N
مهلا
Methyl-4,4-dimethyl-3-oxopentanoate
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
what about this?

Transcribed Image Text:N
مهلا
Methyl-4,4-dimethyl-3-oxopentanoate
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using data from Table 1 plot a graph similar to Figure 4 in the manual that you will label as “Figure A - Bromophenol Blue Stock Solution Absorbance Spectrum”. Remember all graphs need x-y-axis labels and titles. Plot the graph of order 2 polynomial as the best fit curve. The best-fit curve should show the equation of the line, it does not need to pass through the origin (0,0).arrow_forwardPlease help me with this. I will immediately give you a thumb up. Thank you so much! briefly describe the main idea of the variational principal and how it is used in the variational method?arrow_forwardI know you don't answer graded questions and I am not looking for you to straight up give me the answer to this but how do I even start? like I said before I know that the highest peak is 16amu which is Oxygen but do I just the vertical bar as a ratio? Like the next bar to the left (15amu?) is like 90% intensity? so like 9:10 ratio? There isn't even a 15amu element so please just give me some idea where to start. The place in the book it tells you to go to read has nothing about this stuff, its about finding empirical formulas from mass or percentages.... please help me!arrow_forward
- is this a Z or E? and why is it.arrow_forwardcould you check , both answers are wrongarrow_forward7. Don believes that fewer than one-third of the students at his college hold part-time jobs while in school. To investigate this, he randomly surveyed 116 students and found that 32 students held part-time jobs. a. ( - Provide a 90% confidence interval for the population proportion of students that hold a part-time job. b. 1 (a). ;Give the correct interpretation of the interval in part c. Extra Credit: Does this interval support Don's claim? Why?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY