Question
State whether it is true that “the A spectrum is AM0 and the B spectrum is AM1.5”. Explain why B is weaker than A across the whole wavelength range. Show how you estimate the surface temperature of the Sun using the given spectrum.
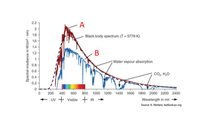
Transcribed Image Text:- A
2.2
2
1.8
Black body spectrum (T = 5778 K)
1.6
1.4
B
1.2
1
Water vapour absorption
0.8
0.6
CO,, H,O
0.4
0.2
200 400
600 800 1000 1200 1400 1600 1800 2000 2200 2400
+ uv + Visible + IR +
Wavelength in nm
Source: K. Mertens: textbook-pv.org
Spectral irradiance in W/(m² . nm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Consider a red photon and a violet photon. a) How do their photon energies compare to one another? b) How do the frequencies of their photons compare to one another? Explain your answer. c) How do the wavelengths of these two photons compare to one another? Explain your answer..arrow_forward2. The wavelength of maximum intensity of the sun's radiation is observed to be near 500 nm. Assume the sun to be a black-body and calculate,imsed of (a) the sun's surface temperature (b) the power per unit area (intensity) emitted from he sun's surface. (c) At what rate is the sun losing mass in units of kg/s? (Hint: What are the units from rignslavaw wert odrai Jari W (d) your answer in part (b)?) (d) At that rate, how much time would it take to exhaust the sun's fuel supply? Does your answer seem reasonable? (The sun's mass is ~2.0x1030 kg)arrow_forwardAnswer all parts pleasearrow_forward
- Which of the following is a feature of blackbody emission spectra? A. The spectrum is discrete B. The spectrum is observed only in the ultraviolet region C. The total radiated power varies as temperature to the second power D. The peak of the curve shifts with temperaturearrow_forwardAnswer all parts pleasearrow_forwardPlease explain and give the correct answerarrow_forward
arrow_back_ios
arrow_forward_ios