
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:%20Fundamentals%20and%20Applications...
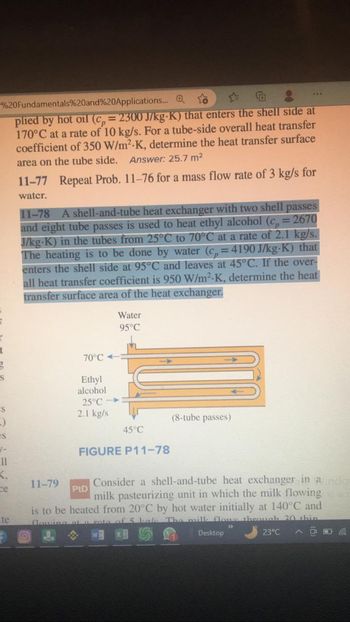
plied by hot oil (cp=2300 J/kg-K) that enters the shell side at
170°C at a rate of 10 kg/s. For a tube-side overall heat transfer
coefficient of 350 W/m²-K, determine the heat transfer surface
area on the tube side. Answer: 25.7 m²
r
t
g
S
S
)
es
_11
K.
ce
te
11-77 Repeat Prob. 11-76 for a mass flow rate of 3 kg/s for
water.
11-78 A shell-and-tube heat exchanger with two shell passes
and eight tube passes is used to heat ethyl alcohol (c, = 2670
J/kg.K) in the tubes from 25°C to 70°C at a rate of 2.1 kg/s.
The heating is to be done by water (c=4190 J/kg-K) that
enters the shell side at 95°C and leaves at 45°C. If the over-
all heat transfer coefficient is 950 W/m².K, determine the heat
transfer surface area of the heat exchanger.
70°C-
11-79
Ethyl
alcohol
25°C
2.1 kg/s
Water
95°C
45°C
FIGURE P11-78
(8-tube passes)
PtD
Consider a shell-and-tube heat exchanger in a indo
milk pasteurizing unit in which the milk flowing
is to be heated from 20°C by hot water initially at 140°C and
flowing at a toto of 5 kale The milk flows through 20 thin
-
23°C ^
Desktop
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Thermal conductivity: 0.53 W/cm C 8. You have been assigned to design a heat exchanger to cool ou a gaseous process stream in a chemical plant. The stream (20 kg/s) enters at 431 K. has a heat capacity of 3.45 J/g C, and needs to be cooled to 402 K. Cooling water is available at 85°F and has a specified maximum temperature of 120 F. The over- all heat-transfer coefficient is approximately 570 W/m² K. How much area must the heat exchanger have? cm 5 cmarrow_forwardOne side of a copper block 5 cm thick is maintained at 250°C. The other side is covered with a layer of fiberglass 2.5 cm thick. The outside of the fiberglass is main- tained at 35°C, and the total heat flow through the copper-fiberglass combination is 52 kW. What is the area of the slab?arrow_forwardA stream of ammonia is cooled from 100oC to 20oC at a rate of 180 kg/hr in the tube side of a double-pipe counter-flow heat exchanger. Water enters the heat exchanger at 10oC at a rate of 250 kg/hr. The outside diameter of the inner tube is 3 cm and the length of the pipe is 7m. Using the log-mean temperature difference, calculate the overall heat transfer coefficient (U) for the heat exchanger. Determine the log-mean temperature difference. Determine the heat transfer coefficient for the heat exchanger. Cp for ammonia is 5234J/kgK and cp for water is 4180J/kgK.arrow_forward
- Formaldehyde at 52.688 bar and 175.65 ℃ passes through a heater-expander and emerges at 39.516 bar and 420.45 ℃. There is no work into or out of the heater, but heat is supplied. Calculate the heat transfer into the heater-expander per mole of formaldehydearrow_forward1. A plastic (k = 0.5 W/m K) pipe carries a coolant at -35 °C with a heat-transfer coefficient of 300 W/m².K. The pipe ID is 3 cm and the OD is 4 cm. The exterior pipe surface is exposed to air at 25°C with a heat-transfer coefficient of 20 W/m²K. Radiative heat transfer may be neglected in this problem. (a) Calculate the rate of heat transfer to the coolant per meter of pipe length. (b) Calculate the temperature of the exterior pipe surface.arrow_forwarda cylinder-plunger contains 2.4x10 ^ -2 kg of air that is subjected to a cycle: Process 1-2: Isochoric process (0.02m ^ 3) from .1MPa to 0.42MPa Process 2-3: isobaric cooling Process 3-1: Isothermal heating Calculate Q and W for each stagearrow_forward
- The air at 20 ° C and 60% RH is heated using a heat exchanger to reach 70 ° C. Using a psychrometric chart, determine a. The amount of heat added per 1 m³ of initial air = (kJ). b. Condensation temperature = (° C)arrow_forward11.22 A shell-and-tube heat exchanger must be designed to heat 2.5 kg/s of water from 15 to 85°C. The heating is to be accomplished by passing hot engine oil, which is available at 160°C, through the shell side of the exchanger. The oil is known to provide an average convection coefficient of h, = 400 W/m² K on the outside of the tubes. Ten tubes pass the water through the shell. Each tube is thin walled, of diameter D = 25 mm, and makes eight passes through the shell. If the oil leaves the exchanger at 100°C, what is its flow rate? How long must the tubes be to accomplish the desired heating?arrow_forward100 kg/s of a crude oil is heated from 24°C to 40°C through the tube side of a multitube heat exchanger. The crude oil flow is divided evenly among all 100 tubes in the tube bundle. The ID of each tube is 10 mm, and the inside tubewall temperature is maintained at 100°C. Average properties of the crude oil are p = 950 kg/m³, cp=1.9 kJ/kg-K, k= 0.25 W/m-K, μ = 12 mPa-s, and Uw = 4 mPa.s. Estimate the rate of heat transfer and the tube length. The rate of heat transfer is The tube length is m. W.arrow_forward
- 03: Air is contained in a vertical cylinder fitted with frictionless piston and a set of stops. The piston cross -sectional area is (0.2 m²) and the air inside is initially at (200 kPa, 500°C). The air is then cooled as a result of heat transfer to the surroundings. a) What is the temperature of the air inside the cylinder when the piston reaches the stops? b) The cooling is now contained until the temperature reaches (20°C), what is the pressure at this state? Ans. [a) 113.4 °C, b) 151.7 kPa] Air 0.25 0.25arrow_forwardA stream of ammonia is cooled from 100oC to 20oC at a rate of 180 kg/hr in the tube side of a double-pipe counter-flow heat exchanger. Water enters the heat exchanger at 10oC at a rate of 250 kg/hr. The outside diameter of the inner tube is 3 cm and the length of the pipe is 7m. Using the log-mean temperature difference, calculate the overall heat transfer coefficient (U) for the heat exchanger. Determine the heat transfer rate between the two fluids. Determine the outlet temperature of the water. Determine the heat transfer surface area. Determine the log-mean temperature difference. Determine the heat transfer coefficient for the heat exchanger. Cp for ammonia is 5234J/kgK and cp for water is 4180J/kgK.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The