
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
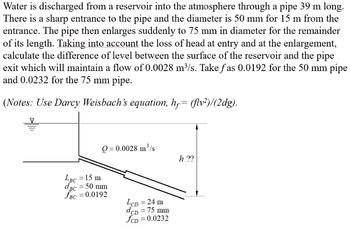
Transcribed Image Text:Water is discharged from a reservoir into the atmosphere through a pipe 39 m long.
There is a sharp entrance to the pipe and the diameter is 50 mm for 15 m from the
entrance. The pipe then enlarges suddenly to 75 mm in diameter for the remainder
of its length. Taking into account the loss of head at entry and at the enlargement,
calculate the difference of level between the surface of the reservoir and the pipe
exit which will maintain a flow of 0.0028 m³/s. Take fas 0.0192 for the 50 mm pipe
and 0.0232 for the 75 mm pipe.
(Notes: Use Darcy Weisbach's equation, h,= (flv²)/(2dg).
Q=0.0028 m³/s
LBC = 15 m
dBc = 50 mm
fBC = 0.0192
LCD = 24 m
dcD = 75 mm
fCD=0.0232
h ??
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 11 images

Knowledge Booster
Similar questions
- 6.39. Two large water tanks are connected by a 10-ft piece of 3-in pipe. The levels in the tanks are equal. When the pressure difference between the tanks is 30 lbf/in², what is the flow rate through the pipe?arrow_forwardIt is desired to have a laminar flow for the piping system shown below. The fluid flowing in the piping system is air at 70 °C. (Note: You must use the physical data from Perry's Handbook) a) Calculate the maximum allowable bulk velocity in m/s for pipes 1, 2, and 3 that will satisfy the statement above. The velocities obtained must satisfy the continuity equation. b) Calculate the Reynolds number of each pipe using the velocity values obtained earlier. c) Calculate the mass flow rate of the system (in kg/min). 3. 2 12-in. pipe 2-in. pipe 3-in. pipe 1/2-in. pipearrow_forwardquestion in imagearrow_forward
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The