
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
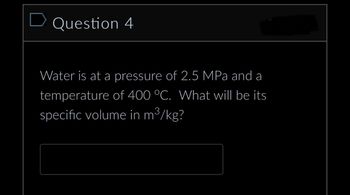
Transcribed Image Text:D Question 4
Water is at a pressure of 2.5 MPa and a
temperature of 400 °C. What will be its
specific volume in m³/kg?
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A gas initially at an absolute pressure of 30 in Hg and 20 °C heat undergoes a process to an absolute pressure of 50 in Hg and 50 °C heat. If after the process the amount of gas is 8 ft. how many cubic inches was it originally?arrow_forwardA cylindrical underground storage tank with the diameter of 18.0 ft and a height of 25.0 ft is filled with gasoline given a density of 675kg/m3 determine the mass of the gas's in the tank in lbm and kg. If the average automobile gas tank holds 25.0 gallon. Calculate the number of autos that may be filled from this facilityarrow_forwardwhat do we do with the atmospheric pressure? which is given to us as 14.7 psiarrow_forward
- A 1 m3 container is filled with 400 kg of granite, 200 kg of dry sand, and 0.2 m3 of liquid 25 °C water. Use properties from Tables A.3 and A.4. Find the average specific volume and density of the masses when you exclude air mass and volume.arrow_forwardA 300 L rigid tank contains Methane gas at 35 °C and 350 kPa. Some methane is allowed to enter the tank tell the until the density in the tank becomes 5.5 kg/m and the temperature at this point is 36 C. 3 What is the amount of gas that has entered the tank (kg)? Select one: a. 1.071 b. 1.131 c. 0.992 d. 0.922 e. None of the abovearrow_forwardThe height of a cylindrical tank containing 15-lb of nitrogen at 70 psia and 115℉ is triple its diameter. Find the tank dimensions in feet.arrow_forward
- 0.3 kg of water fully occupy a closed system 0.15m3 container at 25C. Calculate the specific volume of water Indicate in which state the water is present What is the value of pressure in the container Calculate the quantityarrow_forwardA- Four kg of water is placed in an enclosed volume of 1 m3. Heat is added until the temperature is 150°C. Find (a) the pressure, (b) the mass of vapor, and (c) the volume of the vapor.arrow_forward4. Find the weight of a 50 kg person in N, kgf, lbf, and poundal at standard conditions if 1 kgm 2.20 lbmarrow_forward
- If the specific volume is 0.00075 m³/kg and volume is 0.7 liters. Find the (a) Density (b) Mass, (c) Weight density, and (d) Relative density of the liquid. SOLUTION: (i) Density (in kg/m³) = (ii) Mass of liquid (in gm) = (iii) Weight Density (in N/m³) = (iv) Relative density =arrow_forward2°C 500 //(Kg.°C), is The quantity of heat required to raise the temperature of 500 g of iron by given that the specific heat capacity is 500 kJ 05 kJ 2 KJ O250 KJarrow_forwardDetermine the specific mass in kg/m3 of oil, if the specific volume of the oil is 11.393 x 10-4 m3/kg.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY