
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Solve correctly please.
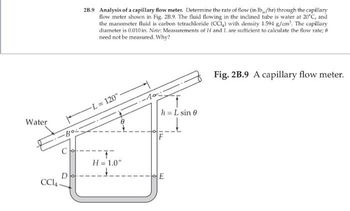
Transcribed Image Text:Water
-Bo
ck
CC14
D
2B.9 Analysis of a capillary flow meter. Determine the rate of flow (in lb/hr) through the capillary
flow meter shown in Fig. 2B.9. The fluid flowing in the inclined tube is water at 20°C, and
the manometer fluid is carbon tetrachloride (CCI) with density 1.594 g/cm³. The capillary
diameter is 0.010 in. Note: Measurements of H and L are sufficient to calculate the flow rate; 8
need not be measured. Why?
L= 120":
T
H = 1.0"
-10:-
h = L sin 0
E
Fig. 2B.9 A capillary flow meter.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- Write detailed short note on PAC.arrow_forwardWhat happens to physical properties in the critical region?arrow_forwardDiagrams need also for this. 2-Electrolyte: The intricate dance of electrons is aided by a liquid bridge filled with a redox mediator between the anode and counter electrode.arrow_forward
- 7.40. The cart in Fig. 7.38 has a mass of 2000 kg. It is resting on frictionless wheels on a solid, level surface and encounters no air resistance. At time zero it is standing still, and a jet from a fire hose is used to start it moving. The mass flow rate of the fluid from the fire hose is 100 kg / s, and its velocity relative to fixed coordinates is 50 m/s. The cup on the rear of the cart turns the jet around so that it leaves in the -x direction with the same velocity relative to the cart with which it entered. Calculate the velocity-time behavior of the cart; assume the jet is unaffected by gravity. (This is not a very practical problem, but it is analogous to the more complex and interesting problem of starting a large turbine from rest. All such turbines must be occasionally shut down for maintenance; their starting and stopping behavior is more complex than their behavior running at a steady speed.)arrow_forwardSolve the percent error columnarrow_forwardGive a brief description of the use of hydrostatic pressure in level measurement. List the main advantages and disadvantages. What is the formula used to deduce level from pressure?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The