
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Mass of Compound A 100 ML

Transcribed Image Text:250 mL Beaker
205.82 mL @ 25.0°C
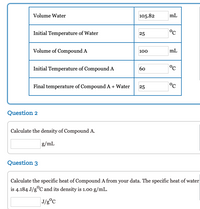
Transcribed Image Text:Volume Water
105.82
mL
Initial Temperature of Water
25
°c
Volume of Compound A
100
mL
Initial Temperature of Compound A
°C
60
Final temperature of Compound A + Water
25
°C
Question 2
Calculate the density of Compound A.
g/mL
Question 3
Calculate the specific heat of Compound A from your data. The specific heat of water
is 4.184 J/g°C and its density is 1.00 g/mL.
J/g°C
Expert Solution
arrow_forward
Step 1
Ques 2) Mass of compound A=100 g
Calculation of the density of compound A:
density=massVolume=100
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a IMG_0299.heic Search What is the molarity of a solution that contains 639 g CuSO4 in 6.00 L of solution? PM In the 'Answer' box, express your numerical answer to 3 significant figures. Give the correct units in the 'units' box. AM Your Answer: AM AM Answer unitsarrow_forwardCalculate the volume in milliliters of a 2.12 mol/L sodium chloride solution that contains 75.0 g of sodium chloride (NaCl). Be sure your answer has the correct number of significant digits. mL Explanation Recheck ** #3 3 80 F3 0.2 X $ 4 F4 S % 5 F5 MacBook Air A A F6 & © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use A F7 00 DII FB ( 9 F9 Privacy Center A 區 F10 ㄷarrow_forwardA 7.00% (m/m) saltwater solution has a density of 1.171 g/mL. How many grams of salt are in 28.00 mL of the saltwater solution?arrow_forward
- Calculate the volume in milliliters of a 0.95 mol/L silver nitrate solution that contains 25.0 g of silver nitrate (AgN0,). Round your answer to 2 significant digits. mLarrow_forwardA 50 g serving of Marshmallow Peeps has 40 g of sugar. What is the % m/m of sugar in Peeps?arrow_forwardCalculate the molarity of each solution. 42.2 mg of KI in 118 mL of solution Express your answer using three significant figures.arrow_forward
- If you were to make a 1/57 dilution with 2.47 mL of sample, how much diluent would you ad 138 mL 141 mL 0.0433 mL 0.0411 mLarrow_forwardWhat is the percent by mass KBr of a solution that contains 88.7 grams of KBr and 119.7 grams of water? Round your answer to 1 decimal. Your Answer: Answer unitsarrow_forward11.38 mg KI in 101.5 mLof solution Express your answer using four significant figures. Molarity = ? Marrow_forward
- Chemistry 64 g of Na2SO4 in 285 mL of Na2SO4 solution. what is mass/volume percent if the bottle of mouthwash contains 370 mL what is the volume in milliliters of the alcohol?arrow_forwardA 5.0 LL sample of a 6.3 MM NaClNaCl solution is diluted to 75 LL . Part A What is the molarity of the diluted solution? Express your answer using two significant figures. nothing MMarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY