
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
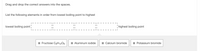
Transcribed Image Text:Drag and drop the correct answers into the spaces.
List the following elements in order from lowest boiling point to highest
lowest boiling point
highest boiling point
:: Fructose C6H12O6
:: Aluminum iodide
:: Calcium bromide
:: Potassium bromide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A heartburn tablet (like Tums or Rolaids) contains food grade calcium carbonate (s) to neutralize stomach acid, HCl(aq). Calcium carbonate is part of the rock cycle in the world of geology and is a type of sedimentary rock called limestone, that forms in oceans. The White Cliffs of Dover England and the Seven Sisters of Sussex England shown in the picture here are also made of calcium carbonate, specifically chalk. Chalk is a pure white limestone formed from the remains of tiny marine organisms (plankton) that lived and died in clear warm seas that covered much of Britain around 70 to 100 million years ago. Do not eat chalk to neutralize stomach acid as it is not purified food grade! Write the balanced molecular equation including phases for solid calcium carbonate reacting with hydrobromic acid. Then write the Full ionic equation including phases. Finally, write the net ionic equation including phases.arrow_forwardGive detailed Solutionarrow_forward27.What is latent heat of condensation? A.the amount of heat released when a certain mass of a substance changes from a gas to a liquid B.the amount of heat that is released when a certain mass of a substance changes from a liquid to a gas C.the amount of heat that is released when a certain volume of a substance changes from a liquid to a gas D.the amount of energy required to raise the temperature of a certain mass of a liquid as it changes from a liquid to a gasarrow_forward
- Draw a picture of and describe with at least one sentence London Dispersion Forces (LDF) between two neon atoms.arrow_forwardA liquid that does not conduct an electrical current although it does dissolve in water is most likely… a) a polar molecular compound b) a nonpolar molecular compound c) an ionic compound d) a metallic substance e) Both c and d are correctarrow_forwardWrite a balanced equation for the formation of potassium chloride with each of the following. (a) potassium bicarbonate and hydrochloric acid (b) potassium carbonate and hydrochloric acidarrow_forward
- Please refer to Figure 1 in filling up Table 1 with increase,decrease or constant Table 1 . Energy involved in the Heating Curve Stage 1 Stage 2 Stage 3 Kinetic Energy Potential Energyarrow_forwardWhen the solid and liquid phase are in equilibrium, which phase, solid or liquid contains h greater amount of energy? Explainarrow_forwardWhy do pennies turn "gold" upon heating? What happens on their surfaces?arrow_forward
- Determine the heat in kJ to heat 7.18 moles of ice from -29.286 degrees celcius to -2.357 degrees celcius. the specific heat of ice is 2.03 J/g degrees celcius.arrow_forwardA substance that could not be chemically divided into a simplier substance is called an?arrow_forwardIn which form of water are the water molecules closest together? (A) liquid water, (B) ICE, (C) Water vaporarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY