
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Is it right? Did I did anything wrong? Is the reaction steps right?
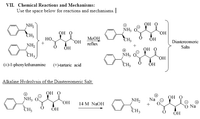
Transcribed Image Text:VII. Chemical Reactions and Mechanisms:
Use the space below for reactions and mechanisms.
Он о
NH,
NH3
он о
но
CH3
MeOH
CH3
ÕH
Но.
Diastereomeric
HO
reflux
Salts
NH2
ÕH
Он О
CH3
NH3
CH3
ÕH
(+)-1-phenylethanamine
(+)-tartaric acid
Alkaline Hydrolysis of the Diastereomeric Salt:
OH O
Он О
Na
NH3
NH2
OH
14 M NaOH
Na
CH3
ÕH
CH3
ОН

Transcribed Image Text:DisSlereomenie
014
CH3
Meo4
cH3
Alkpline Hy doly sis
oF the Digs lene menic splt
1.
క్రి
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the reaction below, what is A[CH,O]/At with respect to A[O2]/A? C,H,(g) + O3(g) → 2CH,0(g) + ½O2(g)arrow_forward1A. Used to determine how the change in concentration will change the rate of reaction.a. General Rate of Reactionb. Rate of Reactionc. Rate Lawd. Order of the Reaction1B. This kind of research does not change the rate even with the various amount of reactant.a. Zero-order reactionb. First-order reactionc. Second-order reactiond. Third-order reaction1C. A condition of equal chemical rate for forward and backward reaction.a. Chemical Equilibriumb. Chemical Balancec. Chemical rate of reactiond. Chemical rate lawarrow_forwardWhich reactions have a positive ASrxn? A(g) + 2B(g) A(s) + B(g) 2 A(g) + 2 B(g) 2 A(g) + 2B(g) 2 C(g) 2 C(g) 3 C(g) 5 C(g)arrow_forward
- The overall reaction #5 is referencing is above (first image). I DO NOT need help with #4, just #5, but 5D has to do with 4. I'm looking for some help with my main part of my question, number 5 A-D, but part 5D also asks for enthalpy values (I guess calculated from my first image)? I think the reaction is second order, but I'm not sure - I think it's second order because of the units Lmol^-1s^-1? And I don't really understand how to sketch a reaction energy diagram...arrow_forwardPlease explainarrow_forwardBelow you will find twelve (12) reaction products (labeled product 1, product 2, etc.), but you will only find five (5) reaction schemes. Your job is to match the reaction scheme with the correct product. Simply fill in the blank with the product number (i.e. 1, 2, 3, etc.). Then, answer any additional questions regarding the reaction scheme(s). Br OH x OMe Product 2 Product 3 Product 4 CH3 x Product 6 Product 10 x Product 1 CO₂H Product 5 Product 7 OMe Product 11 Product 12 Product 9 1. Mgº (metal) a. 2. CO₂ (s) HBr H₂O₂ 3. H3O+ i. What is the regiochemistry of the first reaction? ii. What is the name of the first step of the second reaction? (Hint: he won the 1912 Nobel Prize for this reaction). OMe Br Product 8 CO₂H Brarrow_forward
- True or False (6 - 7) 6. It is found for the reaction A + B → C that doubling A while keeping B constant makes the reaction go 8 times faster and doubling B while keeping A constant makes the reaction go 2 times faster. The rate law for this reaction is: rate= k (A][B]* 7. Suppose you were dissolving a metal such as zinc with hydrochloric acid. Particle size of zinc has no effect on the rate of reaction.arrow_forwardspecies A converts into a product species E. Energy A B Exothermic and A Exothermic and B Exothermic and C A E C 2 D Reaction Progress B is rate determining. C is rate determining. D is rate determining. 3h in which a reactant Is the overall reaction exothermic or endothermic? And which step is the rate determining step? Earrow_forwardew K History Bookmarks DZL Rate Laws Expe X Profiles Tab Determination c X 1 Homework 2 ttempt 1 Listen Window my.edu/d21/le/content/408344/viewContent/17004406/View Help Your Answer: Rates Law Expe X D21 4. CLASS SLIDE X 3 What is the rate constant for a reaction based on the following experimental information? Expt Rate (M/s) [A] (mol/L) [B] (mol/L) 1 0.5170 0.700 0.648 2 1.4912 1.189 0.648 1.2205 1.529 D21 Activity Assignr X 0.700 Report your answer to THREE significant figures. A ALEKS-Isabell x Earrow_forward
- Leaming Goal: • Part A To help you understand how to interpret potential energy diagrams. What is the value of the activation energy of the uncatalyzed reaction? The graphs shown here are called potential energy diagrams. They show the potential energy of a system as it changes from reactants to products. The first graph shows the uncatalyzed reaction (Figure 1); the second graph shows the catalyzed reaction (Figure 2). Express your answer to three significant figures and include the appropriate units. > View Available Hint(s) Symbols undo redo teset keyboard shortcuts Help Tempietes activation energy = |175 kJ Submit Prevlous Answere X Incorrect; Try Again; 5 attempts remaining • Part B What is the value of the enthalpy change of the uncatalyzed reaction? Express your answer to three significant figures and include the appropriate units. Figure O 1 of 2 > • View Available Hint(s) Uncatalyzed Reaction Templetes Symbols undo regdo feset keyboard shortcuts help 125 kJ AH = reactants 200…arrow_forward7, Q1arrow_forwardConstruct the expression for Kc for the following reaction. 4 HCI(aq) + O:(g) = 2 H:O(I) + 2 Cl:(g) 1 Drag the tiles into the numerator or denominator to form the expression. Each reaction participant must be represented by one tile. Do not combine terms. Kc 5 RESET [HCI] 2[HCI] 4[HCI] [HCIP [HCIJ* [0:] 2[0:] 4[O:] [H:O] 2[HŁO] 4[H.O] [H:OJ [CL] 2[CL] 4[CL] [CL*arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY