
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
View and analyze the given spectroscopic data of an unknown compound with molecular formula C8H10O. Propose a structure based on your analysis. Support your answer by interpreting the given spectroscopic data.
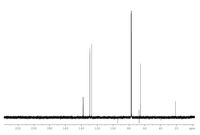
Transcribed Image Text:220
200
180
160
140
120
100
80
60
40
20
ppm
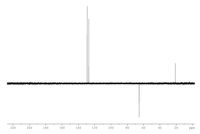
Transcribed Image Text:220
200
180
160
140
120
100
80
60
40
20
ppm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Similar questions
- Calculate AHrxn for the reaction: CH4 (9) +4 Cl(g) → CCl4 (g) + 4 HCl(g) Use the following reactions and given AH´s: C(s) + 2 H₂(g) → CH4 (9) C(s) + 2 Cl₂(g) → CCl4 (9) H₂(g) + Cl₂(g) → 2 HCl(g) AH = -74.4kJ AH = -95.7kJ AH = -92.3 kJarrow_forwardCan molecules with the molecular formulas below exist? (a) CH400 (b) H₂NO (c) C₂HgNarrow_forwardHow many tertiary carbons in this compound? Br OH O 2 0 1 O 5 4.arrow_forward
- HC HC. CH CH CLA Chemical formula: C1arrow_forwardWrite the systematic name of each organic molecule: structure name CH3 CI CH3 CH-CH-CH- CH2 C-OH ☐ CH3 0=0 HO C- -CH-CH2- — CH -CH2 CH3 OH . OH CH3 ཁོང་ལ་ག་ར་པ་ཡི་ཙམ་བཟོད་པ་མ་པ་ CH2-CH-CH-C-OH ☐arrow_forwardFor a given molecular formula of a hydrocarbon, such as C6H14, draw the structural formulae of its different structural isomers. For a given structural isomer, be able to draw several diagrams that all represent the same isomer that has been transformed by (a) rotation of the whole molecule and/or (b) rotation around single covalent bondsarrow_forward
- a) which bond is present in alcohols but not alkanes? b) is this band strong or weak (in terms of spectra)? c) what is different about C=O and O-H bonds compared with C=C or C≡C bonds that show weaker signals?arrow_forwardWhat functional group is evidenced by the IR spectrum shown? carboxylic acid O ketone O ester O aldehydearrow_forwardIdentify whether the following pairs of structures are: (A) Same compound, (B) Constitutional isomers, (C) Cis-trans isomers or (D) Not isomersarrow_forward
- The bonding between the carbon and oxygen in a ketone can be described as C(sp2)-0(sp²), o-bond and C(p)-O(p), л-bond. How would you describe the bonding between carbon and oxygen in a ketene? Sketch the orbitals used to make the bonds in the ketene. H a ketenearrow_forwardWhich line structures represent the molecule in the box? 1110 애 Br (A 오 三 (E 애 애 111 Br Br H Br B B- F Br 애 애 애 Brarrow_forwardDraw 2 possible structures for each of the formulas; C6H8 and C6H6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY