
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
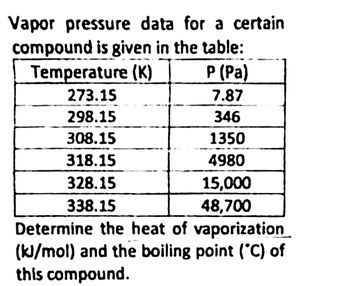
Transcribed Image Text:Vapor pressure data for a certain
compound is given in the table:
Temperature (K)
273.15
298.15
308.15
318.15
328.15
338.15
P (Pa)
7.87
346
1350
4980
15,000
48,700
Determine the heat of vaporization
(kJ/mol) and the boiling point (°C) of
this compound.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 7 images

Knowledge Booster
Similar questions
- Derive (UV), T for VDW gas.arrow_forward37. In measuring the vapor pressure of a liquid by means of the isoteniscope, the height of the Hg in the manometer was found to be 53.32 cm at 40°C, and 39.40 cm at 55°C. The barometric pressure was 741.0 mm. What are the vapor pressures of the liquid at the two temperatures?arrow_forward1 mol N2 gas with a volume of 300 Kelvin and 2 L undergoes an isothermal reversible expansion to the final volume of 20 l. In this case, consider that the gas a) behaves ideally, b) behaves according to the van der Waals equation. Calculate the percentage error in the work when using the ideal gas equation instead of the Van der Waals equation.arrow_forward
- Write the full solution using thermodynamics equation. Calculate the molar density of the saturated liquid and saturated vapor by the soave/redlich/kwong (SRK) equation for n-butane at 110 °C where saturation pressure is 18.66 bar and compare (by % difference) the results with values found by suitable generalized correlations.arrow_forwardSolve the following problems:- 1-How many moles of gas are contained in 890.0 mL at 21.0 °C and 750.0 mm Hg pressure?arrow_forward5- A vacuum gauge indicate a pressure of 0.2 bar of a gas enclosed in a rigid cylinder, the atmospheric pressure is 101.325 kPa, what is the absolute pressure of the gas?arrow_forward
- Match each of the following to the gas it best describes. an realtivity inert gas, that can cause problems if the pressure is very high oxygen a gas essential for life nitrogen a gas that make it more comfortable to [ Choose ] breathe a gas believed to be associated with Climatic change ( Choose a complete inert gas found in normal air argonarrow_forwardQ1) A vessel havinga volume of (0.6 m) contains (3.0 kg) of liquid water and water vapour mixture in equilibrium at a pressure of (0.5 MPa). Calculate: ) Mass and volume of liquid. (ii) Mass and volume of vapour.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The