
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
An adiabatic steam turbine in a small electric power plant is designed to accept 4500lg/hr of steam at 60 bar and 500°C and exhaust the steam at 10 bar.
In off peak hours the power output of the turbine is decreased by adjusting a throttling valve that reduces the turbine inlet stream pressure to 30 bar while keeping the flow rate constant (see Figure below) . What is the turbine inlet temperature, the steam temperature at the outlet of the turbine and the power output of the turbine?
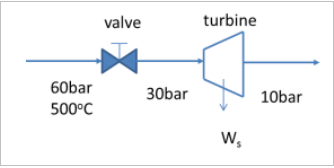
Transcribed Image Text:valve
turbine
60bar
30bar
10bar
500°C
W,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 8arrow_forwardWater vapor expands at a turbine at a speed of 1350 kg / s, the inlet conditions are 8MPA and 400C, the outlet conditions are 500KPa and 250C, the turbine does 200kW work. Neglect changes in kinetic and potential energy. a) Write and simplify the equation that applies to this system. b) What are the specific enthalpies of the input and output current? c) What is the heat transfer in Kw? d) What is the specific volume and volumetric flow at the inlet? e) What is the specific volume and the volumetric flow at the outletarrow_forwardSeparate streams of air and water flow through the compressor and heat exchanger arrangement shown below. Steady-state operating data is provided in the figure. Heat transfer with the environment can be neglected, as can all potential and kinetic energy effects. Air is modeled as an ideal gas. Determine: (a) the total power required by both compressors, in kW. (b) the mass flow rate of the water, in kg / s.arrow_forward
- Q.3 A converging-diverging nozzle is supplied by a huge air reservoir. The nozzle has a throat area of 10 cm? and an exit area of 20 cm?. The reservoir conditions are P. = 300 kPa and To = 30 °C. Determine the range of the back pressure over which (a) shocks appear in the nozzle, (b) oblique shocks form at the exit, and (c) expansion waves form. (d) Calculate the mass flow rate through the nozzle for the case in which a normal shock appears at the exit plane.arrow_forwardQuestion 2 A water pump driven by an 800 W electric motor is used to pump water from the ground level to a tank at the rate of 20000 L/hour. Water is being discharged into the tank at 10 meters above the ground; this is the maximum rise this pump can achieve. The water and the ambient temperatures are both 10 °C. The inlet and outlet pipe diameters for the pump are both 8 cm, respectively. Determine: (1)/ The average inlet linear flow velocity of the water; 2) Assuming the efficiency of the electric motor is 95%, calculate the efficieney-of the motor-pump unit; 3) The pressure difference between the inlet and the outlet of the pump.arrow_forwardAir at 150 kPa and 25°C is transported through a 2-inch Schedule 40 commercial steel pipe. Consider that the air behaves as an ideal gas, that the flow is adiabatic and that the Mach number under these conditions of pressure and temperature is 0.35. Calculate the length of the pipe when the air pressure and temperature decrease to 54.375 kPa and -10.6°C.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The