
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Using the titration data below, plot the titration curve of this unknown diprotic amino acid and determine pKa1. Report your final answer to two places after the decimal.
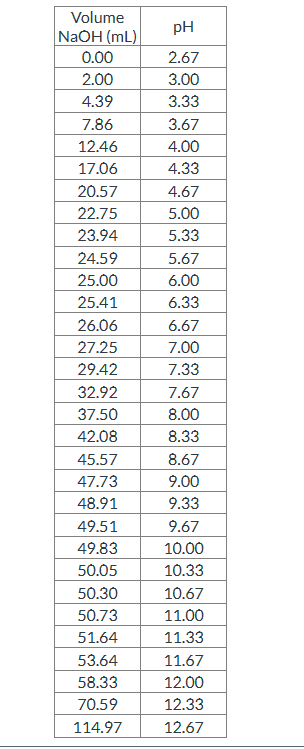
Transcribed Image Text:Volume
NaOH (mL)
0.00
2.00
4.39
7.86
12.46
17.06
20.57
22.75
23.94
24.59
25.00
25.41
26.06
27.25
29.42
32.92
37.50
42.08
45.57
47.73
48.91
49.51
49.83
50.05
50.30
50.73
51.64
53.64
58.33
70.59
114.97
pH
2.67
3.00
3.33
3.67
4.00
4.33
4.67
5.00
5.33
5.67
6.00
6.33
6.67
7.00
7.33
7.67
8.00
8.33
8.67
9.00
9.33
9.67
10.00
10.33
10.67
11.00
11.33
11.67
12.00
12.33
12.67
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the literature value (from Kotz et al) for the Ka for HC3H5O2 (propanoic acid, also written as C2H5CO2H), calculate the theoretical Kb for the C3H5O2- propionate ion and the theoretical pH of a 0.173 M solution of sodium propionate. Using the literature Ka for theCH3NH3+ ion, calculate the theoretical pH of a 0.147 M solution of methylammonium chloride. What is the hydroxide ion concentration? A 0.119 M solution of Covidenol, a weak acid, has a measured pH of 3.45. Determine the pKa of Covidenol.arrow_forwardIdentify the species represented by each curve in the fractional composition diagram of a triprotic acid (H3A) with pKa values of 3.00, 6.00 and 10.00.arrow_forwardPhenolphthalein has a pKa of 9.7 and is colorless in its acid form and pink in its basic form. For pH= 7.7 calculate [In−]/[HIn]. Express your answer using two significant figures.arrow_forward
- 1. Buffers that match the pH of blood and the cytoplasm are commonly required in scientific experiments. If you were studying the effects of lactic acid on muscles and needed to design a buffer that would have a pH of 7.2, what chemical components would you select and in what proportions? Include relevant calculations in your answer. Answer: Submit your completed Assignment to Moodle at the end of this lesson.arrow_forwardThe table below shows the tolerance of different species in a body of water. Critical pH Levels for Aquatic Organisms Animal Critical pH Level Snails 9. Clams 6. Bass 5.5 Crayfishes 5.5 Mayflies 5.5 Trout Salamanders Perch 4.5 Frogs 4. Source: U.S. EPA Using the data from the table above, which of the following statements best identifies and describes the organism or organisms with the smallest tolérance range to a change in pH in an aquatic environment?arrow_forwardWrite the Henderson-Hasselbalch equation for a propanoic acid solution (CH₂CH₂CO₂H, pKa [HA] pH = = 4.352 || pH = 4.874 Answer Bank [A] + log Using the equation to calculate the quotient [A¯]/[HA] at three different pH values. [A-] [HA] [A-] [HA] - o = pka pH = 4.874).arrow_forward
- I need help with this problem Please explain it with step by step explanationarrow_forwardIn the laboratory, a general chemistry student measured the pH of a 0.392 M aqueous solution of hydrocyanic acid to be 4.920. Use the information she obtained to determine the Ka for this acid. Ka(experiment)arrow_forwardIf the pH of the blood of most animals shifts 0.3 units. above or below. the normal range (7.3-7.5), what is going to happen?arrow_forward
- Weak Acids and Buffers Write out the simple equilibrium of a weak acid (HA), labeling the conjugate acid and the conjugate base. ● Write out the Henderson-Hasselbalch equation and use this expression to define the meaning of the characteristic pką value.arrow_forwardDetermine the dissociation constants for the acids. Express the answers in proper scientific notation where appropriate. Acid A: pKa = 5.0 K₂ = a Acid B: pKa = 8.60 Ka = Acid C: pKa = -2.0 Ka =arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY