
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
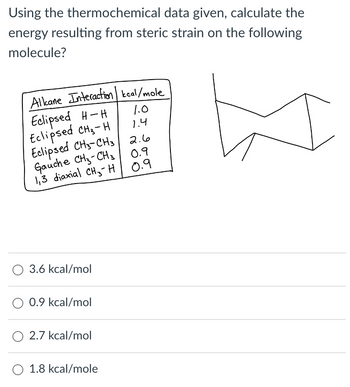
Transcribed Image Text:Using the thermochemical data given, calculate the
energy resulting from steric strain on the following
molecule?
Alkane Interaction kcal/mole
Eclipsed H-H
Eclipsed CH₂-H
Eclipsed CH3-CH3
1.0
1.4
2.6
Gauche CH3-CH3
0.9
1,3 diaxial CH H
0.9
3.6 kcal/mol
○ 0.9 kcal/mol
○ 2.7 kcal/mol
O 1.8 kcal/mole
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Convert to condensed formula: H HTH нн с H-C-C-C НН Н H-c-c-c-c-c-c-c-Н -С-С-С-С-Н НН Н Н Н Н H I I Harrow_forwardComplete the reaction. Add hydrogen atoms and charged to the appropriate atomsarrow_forwardWhich of following structures represents the structural formula for methane, CH? H H-C- Н Н H-C-H H И Н он-с-с-н Он Н C- C H H Нarrow_forward
- For the following two molecules: HO HO O OH HO HO OH NH₂ Isee H₂CO the molecule on the left is sweeter than the molecule on the right these molecules have a bitter taste these molecules have the same level of sweetness the molecule on the right is sweeter than the molecule on the left OHarrow_forward2-propanone, or acetone, can be reduced to 2-propanol by reagents such as lithium aluminum hydride (LiAlH4). Part: 0 / 2 Part 1 of 2 Draw the molecular formula of the product. Click anywhere to draw the first atom of your structure. X :☐ G m 13 Ar B 8arrow_forwardRegarding the three compounds in the previous question which of the following statements is true? Select one: These three compounds are isomers because they have the same molecular formulae. These three compounds are isomers because they have different boiling points. These three compounds are isomers because they have different structures. These three compounds are isomers because they have the same IUPAC name.arrow_forward
- If x is OH in the molecule above, what is the steric strain? Ignore other strain on the molecule, focus on the strain between the x and one hydrogen. 0.5 kJ/mol 0.12 kJ/mol 1.0 kJ/mol 2.1 kJ/molarrow_forwardWhich of the following pairs are identical compounds? CH3 Br and Br CH,OH HOCH CH3 но- OH and OH HO and OH and "OH но HO None of the abovearrow_forwardThe table below shows the boiling point points of an alkane and aldehyde and an alcohol. Explain why the solubility of aldehydes and alcohols falls as the molecules get biggerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY