
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Using the solubility table below, predict the products of the following reaction (Include the states of the products):
NaBr (aq) + AgNO3 (aq) ------>
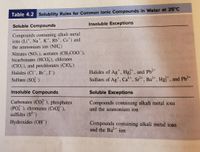
Transcribed Image Text:Table 4.2 Solubility Rules for Common lonic Compounds in Water at 25°C
Insoluble Exceptions
Soluble Compounds
Compounds containing alkali metal
ions (Li*, Na*, K*, Rb*, Cs*) and
the ammonium ion (NH)
Nitrates (NO3), acetates (CH;COO ),
bicarbonates (HCO,), chlorates
(CIO,), and perchlorates (CIO,)
Halides of Ag*, Hg*, and Pb+
Sulfates of Ag", Ca*, Sr*, Ba*, Hg*, and Pb*
Halides (CI, Br, I)
2+
Sulfates (SO)
Insoluble Compounds
Soluble Exceptions
Carbonates (CO?), phosphates
(PO ), chromates (CrO),
sulfides (S-)
Compounds containing alkali metal ions
and the ammonium ion
Hydroxides (OH)
Compounds containing alkali metal ions
and the Ba ion
2+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Good hand written solutionarrow_forwardThe solubility-product constants, Kp, at 25 °C for two compounds [iron(II) carbonate, FeCO3, and cadmium(II) carbonate, CdCO3] are given by the table Substance Кар FeCO3 2.10 x 10-11 CdCO3 1.80 x 10-14 Part A A solution of Na2CO3 is added dropwise to a solution that contains 1.03x10-2 M Fe2+ and 1.52x10-2 MCd2+. What concentration of CO² is need to initiate precipitation? Neglect any volume changes during the addition. Express your answer with the appropriate units. ▸ View Available Hint(s) [CO32] = Value Units Submit Part B Complete previous part(s) Part C Complete previous part(s) Provide Feedback ? Next >arrow_forwardWhat is the molar solubility of calcium hydroxide when it is in the presence of a common ion (CaCl2). The volume of the solution being analyzed is 25mL The volume of HCl added is 19.82mL (this was calculated from minus the final buret reading which was 19.85mL from the initial which was 0.03mL. The concentration of the HCl was 0.0646arrow_forward
- The formation constant* of [M(CN)2]¯ is 5.30 × 1018, where M is a generic metal. A 0.150 mole quantity of M(NO3) is added to a liter of 0.690 M NaCN solution. What is the concentration of M+ ions at equilibrium? [M2+] = M * TOOLS x10arrow_forward32arrow_forwardThe solubility of calcium sulfate at 30°C is 0.209 g/100 mL solution. Calculate its Ksp.arrow_forward
- The solubility product constant for CaCO3 (s) in water is Ksp = [Ca+2] [CO3 -2] = 4.8 x 10^-9 Consider a 1.00 M solution of calcium chloride, in which you drop a piece of Dry Ice. Show that the minimum concentration (threshold amount) of carbonate ions necessary for a precipitate to appear is 4.8 x 10-9 M. Another way to think about this situation is to calculate the Q for the calcium chloride/Dry Ice solution and ask “will precipitation occur?”..arrow_forwardThe molar solubility of silver chromate, Ag₂CrO4, is 1.31×10-4 mol/L. (1) Express the solubility in units of grams per liter. g/L (2) Calculate the concentration of silver ion in a saturated solution of silver chromate. mol/Larrow_forward-4 = A chemistry graduate student is given 500. mL of a 0.20M nitrous acid (HNO₂) solution. Nitrous acid is a weak acid with K = 4.5 × 10 What mass of NaNO₂ should the student dissolve in the HNO₂ solution to turn it into a buffer with pH = 3.46? You may assume that the volume of the solution doesn't change when the NaNO₂ is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. [] x10 Śarrow_forward
- Gallium hydroxide is a very sparingly soluble ionic salt. Given the molar solubility of gallium hydroxide is 7.21 x 10-10 M, what is the Ksp for the dissociation of gallium hydroxide? The pertinent balanced chemical equation is: Ga(OH)3 (s) ↔ Ga3+ (aq) + 3 OH- (aq).arrow_forwardA student carries out the following titration by adding H2SO4.arrow_forward14.5-3 Silver bromide, a solid used in photographic film, can be prepared by mixing solutions of silver nitrate and potassium bromide: Ag+ (aq) + Br(aq) AgBr(s) Keq = 1.87 × 10¹² Calculate the concentrations of Ag and Brions remaining in solution after mixing 2.50 L of 0.100 M AgNO3 solution with 2.50 L of 0.500 M KBr solution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY