
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Pls help ASAP

Transcribed Image Text:Using the reagents below, list in order (by letter, no period) those necessary to transform toluene into
assume any undesired regioisomers can be separated.
Note: Not all spaces provided may be needed. Type "na" in any space where you have no reagent.
A.H₂, Pd
B. I.KMnO4, OH", ii.H3O+
C. CH3COCI, AICI 3
D. Zn(Hg), HCl, reflux
E. i.O3, H₂O ii. Zn, H₂O
F. MMPP, CH3CH₂OH
G. i. BH3; ii. H₂O2, NaOH
L. HCl, peroxide
M. SOCI₂
N. Br2, FeBr3
O. Cl₂, hv
P. Cl2, FeCl3
Q. H₂O, H₂SO4
R. CH3CH₂CH₂CI, AICI3
Br
OH. You may
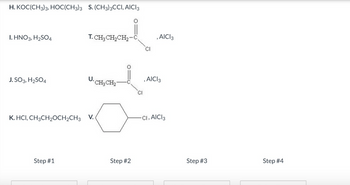
Transcribed Image Text:H.KOC(CH3)3, HOC(CH3)3 S. (CH3)3CCI, AICI 3
1. HNO3, H₂SO4
J. SO3, H₂SO4
T. CH3CH₂CH₂-C
Step #1
U.CH₂CH₂
K. HCI, CH3CH₂CH₂CH3 V.
Step #2
CI
CI
, AICI 3
, AICI 3
CI, AICI 3
Step #3
Step #4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the correct reaction conditions for the following transformation? НО- ОН ОН ? ду ОНarrow_forwardName: (ance eter__ Lab Partners: flat an-kennet_- Name: CODA Section: CHE 1(P Quffers 4. 50 mL of 0.5 M HF is titrated with 25 mL of 0.5 M NaOH. Calculate the pH of the resulting solution A 25 mL solution of 0.1 M Acetic acid (CH3COOH) is titrated with 0.2 M KOH. Calculate the pH after 5.0 mL of KOH have been added. 5. 6. The molar solubility of MnCO3 is 4.2 x 10-6 M. What is the Ksp for this compound?arrow_forwardMCPBAarrow_forward
- What type of linkage in fats is broken by hydrolysis? What synthetic macromolecule contains this type of linkage??arrow_forwardβ-sheets form due to hydrogen bonding between ________, and parallel β-sheets are held together with ________ hydrogen bonds as compared to anti-parallel β-sheets. a side chains; fewer b side chains; more c backbone groups; fewer d side chains; the same number of e backbone groups; more f backbone groups; the same number ofarrow_forwardBr вгик - > Doxarrow_forward
- Which question will your experiment investigate? (select one and delete the others) How does temperature affect how lactase drops work to break down lactose into glucose and galactose? How does pH affect how lactase drops work to break down lactose into glucose and galactose? How does agitation (movement) affect how lactase drops work to break down lactose into glucose and galactose? What variable will you change in this experiment? This is also called the Independent variable. How will you change it? What variable will you measure in this experiment to determine the effect of the variable you changed? This is also called the Dependent variable. How will you measure it? What variables will you keep the same in this experiment? Check off the items in this list that you would use for this experiment. You may add any items you feel you would need. O skim milk (which contains lactose) O Lactase drops O Graduated cylinder Eye dropper Ice cubes O pH paper O Hot plate Mixing spoon O…arrow_forwardProvides the missing structures Br₂/FeBr3 A CH3CH₂CI AICI3 Barrow_forwarduestion 6 of 20 Four major contributing resonance structures are possible for the given cation, which is the intermediate o complex of an electrophilic aromatic substitution involving phenol and bromine. Two structures are given but are incomplete. Complete the given structures by adding nonbonding electrons and formal charges. Draw the remaining structures (in any order), including nonbonding electrons and formal charges. Complete structure A. Complete structure B. Erase Rings More Erase Select Draw Rings More Select Draw |//の H Br Br Br Brarrow_forward
- In addition to have polyalcohol groups, what functional group(s) do monosaccharides have? O a. aldehyde group O b. both ketone and aldehyde groups O C. ether group O d. either ketone or aldehyde group e. ketone group Jump to... You are logged in as Alanie Fontenot (Log out) CHEM 1001/LEC/001X-2021/SPRING/SEMarrow_forwardThanks i need it in words not picturesarrow_forwardneed help with D)Lipidarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY