
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
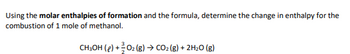
Transcribed Image Text:Using the molar enthalpies of formation and the formula, determine the change in enthalpy for the
combustion of 1 mole of methanol.
CH3OH (e) + O₂ (g) → CO₂ (g) + 2H₂O (g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The compound WO3(s) is utilized in the refining process of tungsten from its mineral ore. Tungsten can be produced as follows: Reaction 1: WO3(s) + 3H2(g) → W(s) + 3H2O(g) a) From the following data calculate the enthalpy of the above reaction Reaction 2: 2W(s) + 3O2(g) → 2WO3(s) ΔH = –1685.4 kJ Reaction 3: 2H2(g) + O2(g) → 2H2O(g) ΔH = –477.84 kJb) Estimate the change of internal energy for Reaction 1. c) Is Reaction 1 exothermic or endothermic?arrow_forwardA chemist measures the enthalpy change AH during the following reaction: COC1₂(9) + 4 NH3(9)→ CO(NH₂),(s) + 2NH4Cl(s) Use this information to complete the table below. Round each of your answers to the nearest kJ/mol. esc COC₁₂ (8) + 2NH₂(g) → CO(NH₂), (s) + NH₂Cl(s) 1 1 ẩCO(NH,),() + ≈NH,C() → † COCI,(8) + NH, (8) CO(NH₂)₂ (s) + 2NH₂Cl(s) → COCL, (g) + 4NH, (g) Explanation THE Check reaction I > AH=-559. kJ Costa ΔΗ kJ ☐ kJ KJ MacBook Pro x10 X S © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acc You MOSISO Larrow_forwardA chemist measures the enthalpy change AH during the following reaction: P4(s) + 6 Cl₂(g) →4 PC1₂(g) AH-1148. kJ Use this information to complete the table below. Round each of your answers to the nearest kJ/mol. reaction PC₁₂ (9) → 4PCL, (g) P₁(s) + 2C₁₂ (9) P₁ (s) + 6Cl₂ (g) P₁ (s) + C₁₂(g) → PCI, (g) ΔΗ [] kJ KJ kJ x10 Xarrow_forward
- A scientist measures the standard enthalpy change for the following reaction to be -19.2 kJ: Ca(OH)2 (aq) + 2HCl(aq) → CaCl₂ (s) + 2H₂O(1) Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of HCl(aq) is kJ/mol. Species AH (kJ/mol) CaCl₂ (8) H₂O(l) Ca(OH)2 (aq) -795.8 -285.8 -1002.8arrow_forward10.00 g of Compound X with molecular formula C4H8 are burned in a constant-pressure calorimeter containing 50.00 kg of water at 25 °C. The temperature of the water is observed to rise by 2.151 °C. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound X at 25 °C. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardA 2.30 g sample of ethanol, CH3CH2OH, is combusted in a bomb calorimeter. The temperature of the calorimeter increases by 4.89 K. If the heat capacity of the bomb is 663 J/K and it contains 2.57 kg of water, what is the enthalpy change per mole of ethanol combusted? The specific heat capacity of water is 4.18 J/g×K and the molar mass of ethanol is 46.07 g/mol.arrow_forward
- The first step in the production of nitric acid from ammonia involves the oxidation of NH3. 4 NH3(g) + 5 O₂ (g) → 4 NO(g) + 6 H₂O(g) 2 a Use standard enthalpies of formation from the table below to calculate the standard enthalpy change for this reaction. Species AfH (kJ/mol) NH3(g) -45.90 NO(g) +90.29 H₂O(g) -241.83 Standard enthalpy change Submit = kJ/mol-rxnarrow_forwardThe combustion of 1.880 g of sucrose, C,H,,O, (s), in a bomb calorimeter with a heat capacity of 4.30 kJ/°C results in an increase in the temperature of the calorimeter and its contents from 22.31 °C to 29.52 °C. What is the internal energy change, AU, for the combustion of 1.880 g of sucrose? AU = kJ Calculate the enthalpy of combustion, AHe, of sucrose in kilojoules per mole. kJ/mol AH. = A étv w MacBook Air DI DD 80 888 F12 F11 F9 F10 F7 FB 3 F4 F5 F6arrow_forwardThe enthalpy change for the following reaction is given below. Pb (s) + CO2 (g) → PbO (s) + CO (g) ΔH° = +131.4 kJ Using the standard enthalpies of formation (∆Hf°) of CO2(g) = −393.5 kJ/mol, and CO(g) = −110.5 kJ/mol, determine the standard enthalpy of formation of PbO(s) A. −151.6 kJ ∕ mol B. −283.0 kJ ∕ mol C. +283.0 kJ ∕ mol D. −372.6 kJ ∕ mol E. +252.1 kJ ∕ molarrow_forward
- Hydrobromic acid (HBr) is a commonly used acid for adding bromine to organic compounds. As bromine makes for an easily replaceable substituent, these bromine precursors are a necessary part of a vast amount of organic eynthesis, Calculate the reaction enthalpy for the synthesis of HBr according to H2(g) + Bra)2Br(g) Use the following information: NH3(g) +HBr(g) → NHABr(s) AH =-188.32 kJ Na(g) + 3H2(g) → 2NH3(g) AH = -92.22 kJ N2(g) + 4H2(g) + Br2(1) → 2NHABr(s) AH =-541.66 kJ a. AH =-728 kmol b. AH =-1010.52 KJjmol C. AH =-826.08 KJ/mol d. AH =728 kJ/mol e. AH = -257.24 kJ/mol Clear my choicearrow_forwardHot water heaters often use natural gas (methane) to generate heat. Methane and oxygen react to form carbon monoxide according to the overall equation: 2CH4(g)+3O2(g) = 2CO(g)+4H2O(g) The enthalpy of formation for each compound are as follows: CH4: - 74.6 O2: 0 CO: - 110.5 H2O: - 285.8 Part A: calculate the overall enthalpy change of the reaction. Part B: A typical water heater contains 40.0 gallons of water. After a long vacation, you come home and turn your water heater from "vacation mode" to hot. How many moles of methane must be combusted to heat the water in your water heater from 30.0°C to 48.0°C? The density of water is 3.79 kg/gal, the specific heat capacity of water is 4.182 J/g°C, and the water heater has a heat capacity of 850.0 J/g°C.arrow_forward(1)Consider the reaction: 2A (g) + 3 B (g) → 2 C (g) ΔHrxn = +254.3 kJ What will be the enthalpy change (in kJ) if 0.471 mol B reacts in excess A? (2)Consider the reaction: C (s) + O2 (g) → CO2 (g) ΔHrxn = -393.5 kJ What mass of carbon (in g) must be reacted via this mechanism to release 581.2 kJ of heat?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY