
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Using the information in the table below, for which of these substances will vaporization be spontaneous at standard temperature and pressure?
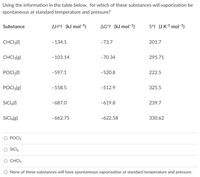
Transcribed Image Text:Using the information in the table below, for which of these substances will vaporization be
spontaneous at standard temperature and pressure?
Substance
AH°f (kJ mol-1)
AG°f (kJ mol-1)
S°f (J K-1 mol-1)
CHCI3(1)
-134.1
-73.7
201.7
CHCI3(g)
-103.14
-70.34
295.71
POCI3()
-597.1
-520.8
222.5
POCI3(g)
-558.5
-512.9
325.5
SICI,(1)
-687.0
-619.8
239.7
SİCIĄ(g)
-662.75
-622.58
330.62
POC13
SiCl4
CHCI3
None of these substances will have spontaneous vaporization at standard temperature and pressure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For benzene, the AH of vaporization is 30.72 kJ/mol and the AS of vaporization is 86.97 J/mol-K. At 1.00 atm and 233.5 K, what is the AG of vaporization for benzene, in kJ/mol?arrow_forwardTitanium tetrachloride is used to create smokescreens over water in naval operations. What is the energy change involved in liquifying 379.4 g solid TiCl4 at its melting point? MP = -23.2 °C AHfus = 9.37 kJ/mol q = [ ? ] kJ Do not round until the end. Include either a + or - sign AND the magnitude. q, (kJ) Enterarrow_forwardThe slope of a plot of In Pvap vs. 1/T (where Tis in kelvin) for a substance is -3774.1 K. What is the enthalpy of vaporization of this substance in kJ/mol? Report your answer with three significant figures. Be sure to include the appropriate unit with your answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY