
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
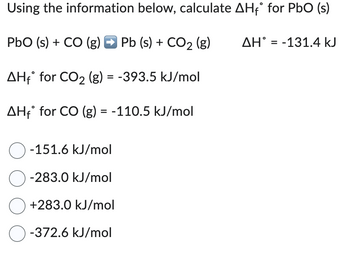
Transcribed Image Text:Using the information below, calculate AH₁° for PbO (s)
PbO (s) + CO (g) → Pb (s) + CO2 (g)
AHf for CO�� (g) = -393.5 kJ/mol
AHf for CO (g) = -110.5 kJ/mol
-151.6 kJ/mol
-283.0 kJ/mol
+283.0 kJ/mol
-372.6 kJ/mol
AH = -131.4 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When 1.42 g of iron reacts with 1.80 g of chlorine, 3.22 g of iron(II) chloride (FeCl2, molar mass = 126.75 g/mol) and 6.8 kJ of heat is produced. What is the enthalpy change for the reaction when 1 mole of iron(II) chloride is produced? round to sig figsarrow_forwardUsing the equations 2 Fe (s) + 3 CI, (g) → 2 FeCl, (s) AH° = -800.0 kJ/mol Si(s) + 2 CI, (g) → SICI, (s) AH° = -640.1 kJ/mol %3D 4 Determine the enthalpy (in kJ/mol) for the reaction 3 SICI, (s) + 4 Fe (s) → 4 FeCl, (s) + 3 Si (s) kJ/molarrow_forwardGiven the following data, DH°f SiCl4(l)= -640.1 kJ/mol, DH°f H2O(l) = -285.8 kJ/mol, DH°f SiO2(s) = -910.9 kJ/mol, DH°f HCl = -92.3 kJ/mol, calculate DH° for the following reaction: SiCl4(l) + 2H2O(l)àSiO2(s) + 4HCl(g) DH° = ? kJarrow_forward
- Find the enthapy change for the reaction below in kJ . 4NH3(g) + 5O2(g) --> 4NO(g) + 6H2O(g) ΔH= ? Given : (NH3(g)) = -46.1, (NO(g)) = + 90.3 kJ/mol and (H2O(g)) = -241.8 kJ/mol.arrow_forward[2] When 1.045 g of CaO is added to 50.0 ml of water at 22.0 °C in a coffee-cup calorimeter, the temperature of the water increases to 29.3 °C. Assume that the specific heat and density of the solution is 4.184 J/g-°C and 1.00g/ml, respectively. The heat capacity of the calorimeter is 18.0 J/°C. Calculate AH for the reaction. CaO(s) + H2O(1) –→ Ca(OH)2(aq); AH =arrow_forwardThe lattice energy is defined as the energy required to break one mole of an ionic solid into its ions in the gas phase. The lattice energy for calcium oxide corresponds to the following reaction. CaO(s) Ca2+(g) + O2-(g) DH = ? Using the following information, determine the lattice energy for calcium oxide. Ca(s) ----> Ca(g) DH = 178.2 kJ Ca(g) ----> Ca2+(g) + 2e- DH = 1747.9 kJ O2(g) ----> 2O(g) DH = 498.3 kJ O2-(g) ----> O(g) + 2e- DH = -702 kJ Ca(s) + 1⁄2 O2(g) ----> CaO(s) DH = -635.0 kJ Using the unrounded answer from the previous problem determine q (kJ) for the following reaction when 1.00 grams of solid calcium oxide forms. Ca2+(g) + O2-(g) ⟶⟶ CaO(s)arrow_forward
- Are the reactions endothermic or exothermic? What observation supports this?arrow_forwardUsing standard heats of formation, calculate the standard enthalpy change for the following reaction. 2HBr(g) + Cl₂(g) → 2HCl(g) + Br₂(g) AH (HBr(g)) = -36.3 kJ/mol AH (Cl₂ (9)) = 0.0 kJ/mol AH (HCl(g)) = -92.3 kJ/mol AH (Br₂ (9)) = 30.9 kJ/mol AH rxn = kJarrow_forwardThe combustion of 0.1566 g benzoic acid increases the temperature of a bomb calorimeter by 2.51°C. Calculate the heat capacity of this calorimeter. (The energy released by combustion of benzoic acid is 26.42 kJ/g.) Heat capacity = kJ/°C A 0.2195-g sample of vanillin (Cg H3 O3) is then burned in the same calorimeter, and the temperature increases by 3.21°C. What is the energy of combustion per gram of vanillin? Energy = kJ/g Per mole of vanillin? Energy = kJ/molarrow_forward
- Using the provided table and the equation below, determine the heat of formation (in kJ/mol) for PbS. 2 PbS (s) + 3 O₂ (g) → 2 SO₂ (g) + 2 PbO (s) AH° = -828.4 kJ/mol Substance O₂ (g) SO: (g) PbO (S) AHf (kJ/mol) 0 -296.9 -217.3arrow_forwardConsider the balanced reaction 4 A + 9 B → 8 C + 8 D and the enthalpies of formation provided in the table below. AH Compound (kJ/mol) A 170.8 231.1 D -98.4 Suppose that the AH of the overall reaction is -2,029.3 kJ/molxn. Calculate the value of the AHf of C in kJ/mol. Report your answer to three decimal places (ignore significant figures).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY