
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
using the image, explain the structure and formation of hydrated ions.
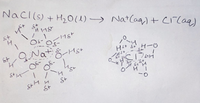
Transcribed Image Text:Naci(s) + H20(1)→ NatCaq)+ CiCaq)
st.
11st
QNat &-Ms+
St
HA* &HーO
StH
St
St
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What happens to sodium carbonate when it dissolves in water?arrow_forwardHow could you distinguish NaCl and Sugar. A By smell B By solubility in water C By color By running reactionsarrow_forwardWrite the balanced net ionic equation for the reaction of aqueous sodium carbonate with aqueous lead(II) nitrate. Include phases. net ionic equation: MacBook Airarrow_forward
- Part of a routine blood test is to check the level of sodium in your blood. It is optimum to have between 135 and 146 micromoles per milliliter [mumol/mL] of sodium. Having an amount too high or too low can cause a variety of health issues. Sodium has a molecular mass of 23 grams per mole [g/mol]. A blood sample is 2.7 [ml]. Determine the minimum mass of sodium that must be present to surpass the upper optimum limit of 146 micromoles per milliliter [mu mol/mL].arrow_forwardTo what volume (in mL) must 60.0 mL of 1.75 M HCl be diluted to produce 0.500 M HCl?arrow_forwardWrite and explain step by step. If you are handwriting it make sure its readible.arrow_forward
- Write out the chemical equations that are involved for:a) the formation of the salt (give the chemical structure for the salt formed)b) the conversion of the salt back to the original compoundarrow_forwardQuestion 9 A student is attempting to identify the anion in an unknown solid. The student first dissolves the solid in aqueous solution, and then adds aqueous barium nitrate. A solid product forms. The student then adds hydrochloric acid, which causes the solid to dissolve. Which of the following anions might be in this unknown sample? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a Phosphate Nitrate Carbonate d Sulfate e Chloridearrow_forwardWhat is an electrolyte? A substance that dissolves in water but doesn't produce ions. A substance that dissolves in water and produces ions. A substance that doesn't dissolve in water. A substance that melts at room temperature.arrow_forward
- Is the acid-base neutralization reaction between sodium hydroxide and hydrochloric acid an exothermic or endothermic process?arrow_forwardSuppose each student in a laboratory prepares two aqueous solutions, Solution A and Solution B, then measures the conductivity of each. Predict the comparison of conductivity between the two solutions. Solution A contains 1.0 moles of MgCl, Solution B contains 1.0 moles of NaOH Choose... Solution A contains 1.5 moles of CH,06 Solution B contains 1.5 moles of NaCl. Choose... Solution A is 10.0 mL of 0.1 M ethylene glycol. Solution B is 25.0 mL of 0.1 M ethylene glycol. Choose..arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY