
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Using the following Latimar diagram, calculate the value that would be plotted on the Frost diagram for the NH4+ species.
Have your answer to 2 decimal places.
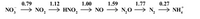
Transcribed Image Text:0.79
1.12
1.00
1.59
1.77
0.27
NO, → NO, –→ HNO, → NO → N,0 → N, → NH
Expert Solution
arrow_forward
Step 1

Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You are asked to make spaghetti. You start boiling the water and find it is taking too long. Your friend suggests that you add salt to the water to speed up the boiling process. a. Based upon the circumstances above, come up with a very specific question you will try to answer through experimentatior b. Based upon knowledge of intermolecular and intramolecular bonding, hypothesize an answer to the question you created. c. Based upon your knowledge of inter and intramolecular bonding, give reasons for your hypothesis. Include diagrams in your explanation d. Design a fair experiment to test your hypothesisarrow_forwardCalculate the value of the force constant for a crystal of sodium chloride if you know that the lattice constant is 2.82 * 10^-10 m, the mass of sodium is 3.8 * 10^-23 gm, the mass of chlorine is 5.9 * 10-23 gm, and their phase speed is 3.4 * 10^-3 m/sec equal to 3.4x6arrow_forwardThe diagram shown below is a theoretical representation of the phase diagram for carbon. The hatched regions indicate conditions under which the phase is predicted to be metastable in effect this means that multiple phases can co-exist in these regions. Using this diagram, answer the following questions concerning the phases of carbon. Note the indicated scales for the horizontal and vertical axes (1 bar = 0.0001 GPa). 1000- 100- p/GPa 10- 0.1 0.01- 0.001+ 0 diamond graphite 1 2 3 4 liquid vapor 5 6 7 T/1000 K 8 9 10 (a) Does graphite melt or sublime at 10 bars of pressure? (b) If pressure remains constant, what are the possible phase transitions for diamond at 4500 K as T is increased? (c) Identify the approximate temperature and pressure of the standard solid-liquid-gas triple point on this diagram. Are there other triple points indicated in the diagram? (d) Estimate the maximum temperature at which graphite can exist at P = 1 GPa. What would happen if the pressure is increased for a…arrow_forward
- Please help me With an in-depth explanationarrow_forwardWhat type of melting point range is expected for a pure solid? What type of melting point range is expecting for an impure solid?arrow_forwardImage: Graph, Energy on X axis, Temp on Y axis. There is a line sloping up and to the right labelled "A" beginning near bottom of Y-axis. At end of this line, there is a horizontal line labelled "B". At the end of this line there is another line sloping up and to the right labelled "C". End of Image. NH3 (MW = 17.034 g/mol) Melting point = minus 77.73 degrees C Boiling point = minus 33.34 degrees C Heat capacity of liquid = 4.700 J/g/K Heat capacity of gas = 2.060 J/g/K Heat of vaporization = 23.40 kJ/mol Find A, B and C (as far as 0 degrees C) and the total energy required (in that order) in Joules (express as 'J'). Assume 100.0 g of substance. A, B and C should have four significant figures. When entering your values import your answer followed by a space then J Example: 3450. J Notice that in this particular example a decimal point was included after 3450 to indicate that the zero is significant. If the answer does not need a decimal do not include one. 1.____ 2.____ 3.____ 4.____arrow_forward
- London dispersion forces, or simply dispersion forces are the from surrounding intermolecular force. It is an attractive force that arises dipole moments in molecules or species. These moments arise when there are instantaneous deviations in the electron clouds of the species. Surrounding molecules are dipole influenced by these dipole moments and a chain reaction results in which subsequent weak, dipole-induced dipole interactions are created. These cumulative dipole-induced dipole interactions create the attractive dispersion forces. Dispersion forces are the forces that make substances condense to liquids and freeze into solids as temperatures decrease. Your answer choices are: weakest, temporary and nonpolar. Several of your choices are used more than once.arrow_forwardNonearrow_forwardHi! I specifically need help with question 4, thank you!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY