
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
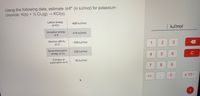
Transcribed Image Text:Using the following data, estimate AHf° (in kJ/mol) for potassium
chloride: K(s) + ½ Cl2(g) → KCI(s).
->
Lattice energy
-690 kJ/mol
of KCI
kJ/mol
lonization energy
419 kJ/mol
of K
Electron affinity
-349 kJ/mol
of CI
1
3
Bond dissociation
239 kJ/mol
energy of Cl2
6.
C
Enthalpy of
sublimation for K
90 kJ/mol
7
8.
9.
+/-
х 100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1. An impure sample of compound A is contaminated with two impurities B and C. The sample is to be purified by recrystallization using ethanol as the solvent. The solubility properties of the three components are summarized below. Solubility in Solubility in ethanol Solubility in 50 mL Solubility in 50 mL ethanol at -78 °C at - 0°C ethanol at -78 °C ethanol at - 0°C (g) (g) Compound A 0.12 g/mL 0.02 g/mL Impurity B 0.58 g/mL 0.04 g/mL Impurity C 0.005 g/mL 0.0003 g/mL The impure (7.5 g) sample contains 5.0 g of compound A, 1.5 g of B and 1.0 g of C and is recrystallized using 50 mL of ethanol. The sample is boiled with 50 mL of ethanol, filtered by gravity and then cooled in ice and filtered by suction. a) How much compound A should be obtained as the final product? Will the sample be contaminated with any of the impurities? Explain (using calculations to support your answer-fill in the missing masses in the table above). Hint: For this question you should calculate the mass of each…arrow_forwardMolecular bromine is 24 percent dissociated at 1600 K and 1.00 bar in the equilibrium Br₂(g) ⇒ 2Br(g). Calculate (a) Kp, (b) A,Gº, (c) K₂ at 2000 K given that A₁HⓇ = +112 kJ mol-¹ over the temperature range.arrow_forwardMn in groundwaterA ground water has the following conditions:{Mn2+ (aq)} = 1.0x10 -5pH = 7.0 (i.e., {H+ } = 1.0x10-7)Saturated with oxygen from the atmosphere: {O 2(aq)} = 2.63x10 -4T = 25 °C(a) Write the balanced equation for oxidation of reduced manganese,Mn2+ (aq), by dissolved oxygen (O 2(aq)) to generate MnO 2(s)(manganese dioxide) or MnOOH(s) (manganite), respectively.(b) Determine whether each of the above two reactions isthermodynamically favorable in the ground water. Which phase ofthe manganese oxide is more stable in the ground water?(c) If groundwater is at 5 oC, does oxidation of Mn 2+ (aq) by dissolvedoxygen to generate MnO 2(s) become more or less favorable?Calculate the saturated {Mn 2+ (aq)} assuming the water pH remainsat 7.0 and the dissolved oxygen concentration is 3.73x10 -4 M. 25C and 1 atm Please be as detailed as possible. I want to be able to replicate. Thank you so much. :)arrow_forward
- How many electrons are transferred in the following process, given the unbalanced reaction? PbO2 (s) + H* (aq) + Fe (s) → Fe3+(aq) + Pb2+ (aq) + H20 (1) Group of answer choices A-1 В-2 C-6 D-4 Е-3arrow_forwardEthylene gas is converted ethanol compound by vapor phase hydration reaction. is being transformed. Maximum conversion amount at 523 K and 35 bar conditions, Calculate based on the amount of steam being 5 times the amount of ethylene. gas in equilibrium Assume that the mixture exhibits ideal solution behavior.arrow_forwardCan you help me with part d?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The